A-867744Allosteric Modulator CAS# 1000279-69-5 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SD-208
Catalog No.:BCC1938
CAS No.:627536-09-8
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1000279-69-5 | SDF | Download SDF |
| PubChem ID | 23642319 | Appearance | Powder |
| Formula | C20H19ClN2O3S | M.Wt | 402.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (248.21 mM) *"≥" means soluble, but saturation unknown. | ||
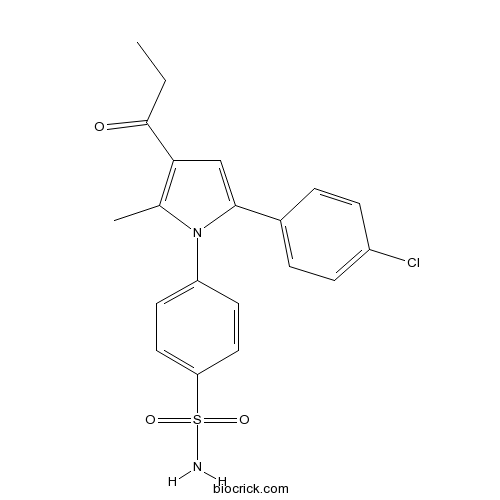
| Chemical Name | 4-[5-(4-chlorophenyl)-2-methyl-3-propanoylpyrrol-1-yl]benzenesulfonamide | ||
| SMILES | CCC(=O)C1=C(N(C(=C1)C2=CC=C(C=C2)Cl)C3=CC=C(C=C3)S(=O)(=O)N)C | ||
| Standard InChIKey | ABACVOXFUHDKNZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19ClN2O3S/c1-3-20(24)18-12-19(14-4-6-15(21)7-5-14)23(13(18)2)16-8-10-17(11-9-16)27(22,25)26/h4-12H,3H2,1-2H3,(H2,22,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Positive allosteric modulator of α7 nAChRs (IC50 values are 0.98 and 1.12 μM for human and rat α7 receptor ACh-evoked currents respectively, in X. laevis oocytes). Displays no activity at 5-HT3A, α3β4 or α4β2 nAChRs. Brain penetrant. |

A-867744 Dilution Calculator

A-867744 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4821 mL | 12.4103 mL | 24.8207 mL | 49.6413 mL | 62.0517 mL |
| 5 mM | 0.4964 mL | 2.4821 mL | 4.9641 mL | 9.9283 mL | 12.4103 mL |
| 10 mM | 0.2482 mL | 1.241 mL | 2.4821 mL | 4.9641 mL | 6.2052 mL |
| 50 mM | 0.0496 mL | 0.2482 mL | 0.4964 mL | 0.9928 mL | 1.241 mL |
| 100 mM | 0.0248 mL | 0.1241 mL | 0.2482 mL | 0.4964 mL | 0.6205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Targeting α7 neuronal acetylcholine receptors (nAChRs) with selective agonists and positive allosteric modulators (PAMs) is considered a therapeutic approach for managing cognitive deficits in schizophrenia and Alzheimer’s disease. A-867744 is a novel type II PAM with good potency and selectivity.
In vitro: In oocytes expressing α7 nAChRs, A-867744 potentiated acetylcholine (ACh)-evoked currents, with an EC50 value of about 1 μM. At highest concentrations of A-867744 tested, ACh-evoked currents were essentially nondecaying. At lower concentrations, no evidence of a distinct secondary component was evident in contrast to 4-naphthalen-1-yl-3a,4,5,9b-tetrahydro -3H-cyclopent-a[c]quinoline-8-sulfonic acid amide (TQS), another type II α7 PAM [1].
In vivo: A-867744 was assessed for its PK properties in rat, dog, and monkey. The animal pharmacokinetic profile of A-867744 was characterized by relatively low plasma clearance values (1.1-2.5 L/h/kg) and volumes of distribution (1.7-4.6 L/kg) across species, with terminal elimination halflives in the 1.0-1.7 h. The bioavailability is 83% in rat and somewhat lower in dog (55%) and monkey (68%). These findings indicated that A-867744 showed acceptable pharmacokinetic profile across species and brain levels sufficient to modulate α7 nAChRs [2].
Clinical trial: A-867744 is currently in the preclinical developlent stage and no clinical data are available.
Reference:
[1] Malysz J, Grønlien JH, Anderson DJ, Håkerud M, Thorin-Hagene K, Ween H, Wetterstrand C, Briggs CA, Faghih R, Bunnelle WH, Gopalakrishnan M.
In vitro pharmacological characterization of a novel allosteric modulator of alpha 7 neuronal acetylcholine receptor, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl) (A-867744), exhibiting unique pharmacological profile. J Pharmacol Exp Ther. 2009;330(1):257-67.
[2] Faghih R, Gopalakrishnan SM, Gronlien JH, Malysz J, Briggs CA, Wetterstrand C, Ween H, Curtis MP, Sarris KA, Gfesser GA, El-Kouhen R, Robb HM, Radek RJ, Marsh KC, Bunnelle WH, Gopalakrishnan M. Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl) benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J Med Chem. 2009;52(10):3377-84.
- 2-Methylthioadenosine triphosphate tetrasodium salt
Catalog No.:BCC6918
CAS No.:100020-57-3
- Dauriporphinoline
Catalog No.:BCN7901
CAS No.:100009-82-3
- Anisole
Catalog No.:BCN2619
CAS No.:100-66-3
- Benzaldehyde
Catalog No.:BCN8529
CAS No.:100-52-7
- Benzylamine
Catalog No.:BCN1789
CAS No.:100-46-9
- Pentamidine
Catalog No.:BCC3836
CAS No.:100-33-4
- 4-Methoxybenzoic acid
Catalog No.:BCN3838
CAS No.:100-09-4
- GIP (human)
Catalog No.:BCC5870
CAS No.:100040-31-1
- TAK-875
Catalog No.:BCC3702
CAS No.:1000413-72-8
- 8-Hydroxy-3,5,6,7,3',4'-hexamethoxyflavone
Catalog No.:BCN7870
CAS No.:1000415-56-4
- AS 19
Catalog No.:BCC7218
CAS No.:1000578-26-6
- KW 2449
Catalog No.:BCC2179
CAS No.:1000669-72-6
- Stilbostemin N
Catalog No.:BCN4741
CAS No.:1000676-45-8
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- 7-Hydroxy-2',5,8-trimethoxyflavanone
Catalog No.:BCN5817
CAS No.:100079-34-3
- 2',4'-Dihydroxy-2,3',6'-trimethoxychalcone
Catalog No.:BCN1643
CAS No.:100079-39-8
- H-Lys(Tfa)-OH
Catalog No.:BCC2985
CAS No.:10009-20-8
- Chloramultilide B
Catalog No.:BCN6613
CAS No.:1000995-47-0
- Chloramultilide C
Catalog No.:BCN6618
CAS No.:1000995-48-1
In vitro pharmacological characterization of a novel allosteric modulator of alpha 7 neuronal acetylcholine receptor, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744), exhibiting unique pharmacological profile.[Pubmed:19389923]
J Pharmacol Exp Ther. 2009 Jul;330(1):257-67.
Targeting alpha7 neuronal acetylcholine receptors (nAChRs) with selective agonists and positive allosteric modulators (PAMs) is considered a therapeutic approach for managing cognitive deficits in schizophrenia and Alzheimer's disease. In this study, we describe a novel type II alpha7 PAM, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744), that exhibits a unique pharmacological profile. In oocytes expressing alpha7 nAChRs, A-867744 potentiated acetylcholine (ACh)-evoked currents, with an EC(50) value of approximately 1 microM. At highest concentrations of A-867744 tested, ACh-evoked currents were essentially nondecaying. At lower concentrations, no evidence of a distinct secondary component was evident in contrast to 4-naphthalen-1-yl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonic acid amide (TQS), another type II alpha7 PAM. In the presence of A-867744, ACh concentration responses were potentiated by increases in potency, Hill slope, and maximal efficacy. When examined in rat hippocampus CA1 stratum radiatum interneurons or dentate gyrus granule cells, A-867744 (10 microM) increased choline-evoked alpha7 currents and recovery from inhibition/desensitization, and enhanced spontaneous inhibitory postsynaptic current activity. A-867744, like other alpha7 PAMs tested [1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro-5-trifluoromethyl-phenyl)urea (NS1738), TQS, and 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea (PNU-120596)], did not displace the binding of [(3)H]methyllycaconitine to rat cortex alpha7(*) nAChRs. However, unlike these PAMs, A-867744 displaced the binding of the agonist [(3)H](1S,4S)-2,2-dimethyl-5-(6-phenylpyridazin-3-yl)-5-aza-2-azoniabicyclo[2.2.1 ]heptane (A-585539) in rat cortex, with a K(i) value of 23 nM. A-867744 neither increased agonist-evoked responses nor displaced the binding of [(3)H]A-585539 in an alpha7/5-hydroxytryptamine(3) (alpha7/5-HT(3)) chimera, suggesting an interaction distinct from the alpha7 N terminus or M2-3 loop. In addition, A-867744 failed to potentiate responses mediated by 5-HT(3A) or alpha3beta4 and alpha4beta2 nAChRs. In summary, this study identifies a novel and selective alpha7 PAM showing activity at recombinant and native alpha7 nAChRs exhibiting a unique pharmacological interaction with the receptor.
Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor.[Pubmed:19419141]
J Med Chem. 2009 May 28;52(10):3377-84.
The discovery of a series of pyrrole-sulfonamides as positive allosteric modulators (PAM) of alpha7 nAChRs is described. Optimization of this series led to the identification of 19 (A-867744), a novel type II PAM with good potency and selectivity. Compound 19 showed acceptable pharmacokinetic profile across species and brain levels sufficient to modulate alpha7 nAChRs. In a rodent model of sensory gating, 19 normalized gating deficits. These results suggest that 19 represents a novel class of molecules capable of allosteric modulation of the alpha7 nAChRs.


