PentamidineDrug to treat protozoal diseases CAS# 100-33-4 |
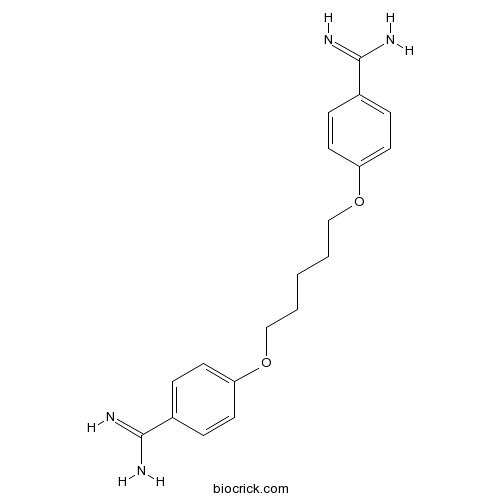
- Pentamidine
Catalog No.:BCC3836
CAS No.:100-33-4
- Pentamidine dihydrochloride
Catalog No.:BCC5194
CAS No.:50357-45-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100-33-4 | SDF | Download SDF |
| PubChem ID | 4735 | Appearance | Powder |
| Formula | C19H24N4O2 | M.Wt | 340.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in sterile water | ||
| Chemical Name | 4-[5-(4-carbamimidoylphenoxy)pentoxy]benzenecarboximidamide | ||
| SMILES | C1=CC(=CC=C1C(=N)N)OCCCCCOC2=CC=C(C=C2)C(=N)N | ||
| Standard InChIKey | XDRYMKDFEDOLFX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H24N4O2/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pentamidine Dilution Calculator

Pentamidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9375 mL | 14.6877 mL | 29.3755 mL | 58.751 mL | 73.4387 mL |
| 5 mM | 0.5875 mL | 2.9375 mL | 5.8751 mL | 11.7502 mL | 14.6877 mL |
| 10 mM | 0.2938 mL | 1.4688 mL | 2.9375 mL | 5.8751 mL | 7.3439 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.175 mL | 1.4688 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5875 mL | 0.7344 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Used as a drug to treat protozoal diseases, such as amoebic dysentery, malaria and trypanosomiasis. Also has been shown to be effective for both prophylaxis of pneumocystic carinii pneumonia (PCC).
- 4-Methoxybenzoic acid
Catalog No.:BCN3838
CAS No.:100-09-4
- Benzylamine
Catalog No.:BCN1789
CAS No.:100-46-9
- Benzaldehyde
Catalog No.:BCN8529
CAS No.:100-52-7
- Anisole
Catalog No.:BCN2619
CAS No.:100-66-3
- Dauriporphinoline
Catalog No.:BCN7901
CAS No.:100009-82-3
- 2-Methylthioadenosine triphosphate tetrasodium salt
Catalog No.:BCC6918
CAS No.:100020-57-3
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- GIP (human)
Catalog No.:BCC5870
CAS No.:100040-31-1
- TAK-875
Catalog No.:BCC3702
CAS No.:1000413-72-8
- 8-Hydroxy-3,5,6,7,3',4'-hexamethoxyflavone
Catalog No.:BCN7870
CAS No.:1000415-56-4
- AS 19
Catalog No.:BCC7218
CAS No.:1000578-26-6
- KW 2449
Catalog No.:BCC2179
CAS No.:1000669-72-6
- Stilbostemin N
Catalog No.:BCN4741
CAS No.:1000676-45-8
Organic cation transporter 1 (OCT1) is involved in pentamidine transport at the human and mouse blood-brain barrier (BBB).[Pubmed:28362799]
PLoS One. 2017 Mar 31;12(3):e0173474.
Pentamidine is an effective trypanocidal drug used against stage 1 Human African Trypanosomiasis (HAT). At the blood-brain barrier (BBB), it accumulates inside the endothelial cells but has limited entry into the brain. This study examined transporters involved in Pentamidine transport at the human and mouse BBB using hCMEC/D3 and bEnd.3 cell lines, respectively. Results revealed that both cell lines expressed the organic cation transporters (OCT1, OCT2 and OCT3), however, P-gp was only expressed in hCMEC/D3 cells. Polarised expression of OCT1 was also observed. Functional assays found that ATP depletion significantly increased [3H]Pentamidine accumulation in hCMEC/D3 cells (***p<0.001) but not in bEnd.3 cells. Incubation with unlabelled Pentamidine significantly decreased accumulation in hCMEC/D3 and bEnd.3 cells after 120 minutes (***p<0.001). Treating both cell lines with haloperidol and amantadine also decreased [3H]Pentamidine accumulation significantly (***p<0.001 and **p<0.01 respectively). However, prazosin treatment decreased [3H]Pentamidine accumulation only in hCMEC/D3 cells (*p<0.05), and not bEnd.3 cells. Furthermore, the presence of OCTN, MATE, PMAT, ENT or CNT inhibitors/substrates had no significant effect on the accumulation of [3H]Pentamidine in both cell lines. From the data, we conclude that Pentamidine interacts with multiple transporters, is taken into brain endothelial cells by OCT1 transporter and is extruded into the blood by ATP-dependent mechanisms. These interactions along with the predominant presence of OCT1 in the luminal membrane of the BBB contribute to the limited entry of Pentamidine into the brain. This information is of key importance to the development of Pentamidine based combination therapies which could be used to treat CNS stage HAT by improving CNS delivery, efficacy against trypanosomes and safety profile of Pentamidine.
The use of intravenous pentamidine for the prophylaxis of Pneumocystis pneumonia in pediatric patients.[Pubmed:28074607]
Pediatr Blood Cancer. 2017 Aug;64(8).
Pneumocystis jiroveci pneumonia was common in the immunocompromised host before the widespread use of prophylaxis. When trimethoprim-sulfamethoxazole is not tolerated, prophylaxis with intravenous Pentamidine (IVP) may be initiated. We performed a retrospective analysis of all pediatric patients who received IVP regarding efficacy, safety, and reason for initiation. Of 106 patients included in our analysis, one patient tested positive for Pneumocystis DNA. Adverse events were reported in 18% of IVP courses, and main reason for initiation was cytopenia (59%). We found IVP to be effective and safe, and recommend the use of IVP in pediatric patients in whom first-line prophylaxis is contraindicated.
Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance.[Pubmed:28263303]
Nat Microbiol. 2017 Mar 6;2:17028.
The increasing use of polymyxins(1) in addition to the dissemination of plasmid-borne colistin resistance threatens to cause a serious breach in our last line of defence against multidrug-resistant Gram-negative pathogens, and heralds the emergence of truly pan-resistant infections. Colistin resistance often arises through covalent modification of lipid A with cationic residues such as phosphoethanolamine-as is mediated by Mcr-1 (ref. 2)-which reduce the affinity of polymyxins for lipopolysaccharide(3). Thus, new strategies are needed to address the rapidly diminishing number of treatment options for Gram-negative infections(4). The difficulty in eradicating Gram-negative bacteria is largely due to their highly impermeable outer membrane, which serves as a barrier to many otherwise effective antibiotics(5). Here, we describe an unconventional screening platform designed to enrich for non-lethal, outer-membrane-active compounds with potential as adjuvants for conventional antibiotics. This approach identified the antiprotozoal drug Pentamidine(6) as an effective perturbant of the Gram-negative outer membrane through its interaction with lipopolysaccharide. Pentamidine displayed synergy with antibiotics typically restricted to Gram-positive bacteria, yielding effective drug combinations with activity against a wide range of Gram-negative pathogens in vitro, and against systemic Acinetobacter baumannii infections in mice. Notably, the adjuvant activity of Pentamidine persisted in polymyxin-resistant bacteria in vitro and in vivo. Overall, Pentamidine and its structural analogues represent unexploited molecules for the treatment of Gram-negative infections, particularly those having acquired polymyxin resistance determinants.
Pentamidine antagonizes the benznidazole's effect in vitro, and lacks of synergy in vivo: Implications about the polyamine transport as an anti-Trypanosoma cruzi target.[Pubmed:27729250]
Exp Parasitol. 2016 Dec;171:23-32.
Benznidazole is the first-line drug used in treating Chagas disease, which is caused by the parasite Trypanosoma cruzi (T. cruzi). However, benznidazole has limited efficacy and several adverse reactions. Pentamidine is an antiprotozoal drug used in the treatment of leishmaniasis and African trypanosomiasis. In T. cruzi, Pentamidine blocks the transport of putrescine, a precursor of trypanothione, which constitutes an essential molecule in the resistance of T. cruzi to benznidazole. In the present study, we describe the effect of the combination of benznidazole and Pentamidine on isolated parasites, mammalian cells and in mice infected with T. cruzi. In isolated trypomastigotes, we performed a dose-matrix scheme of combinations, where Pentamidine antagonized the effect of benznidazole, mainly at concentrations below the EC50 of Pentamidine. In T. cruzi-infected mammalian cells, Pentamidine reversed the effect of benznidazole (measured by qPCR). In comparison, in infected BALB/c mice, Pentamidine failed to get synergy with benznidazole, measured on mice survival, parasitemia and amastigote nest quantification. To further explain the in vitro antagonism, we explored whether Pentamidine affects intracellular trypanothione levels, however, Pentamidine produced no change in trypanothione concentrations. Finally, the T. cruzi polyamine permease (TcPAT12) was overexpressed in epimastigotes, showing that Pentamidine has the same trypanocidal effect, independently of transporter expression levels. These results suggest that, in spite of the high potency in the putrescine transport blockade, TcPAT12 permease is not the main target of Pentamidine, and could explain the lack of synergism between Pentamidine and benznidazole.


