DauriporphinolineCAS# 100009-82-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100009-82-3 | SDF | Download SDF |
| PubChem ID | 68232932 | Appearance | Powder |
| Formula | C19H15NO5 | M.Wt | 337.33 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
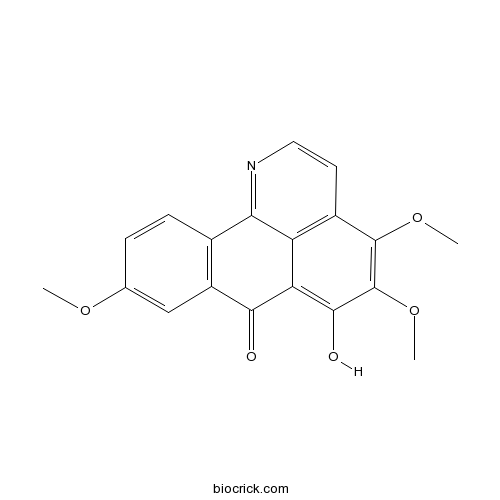
| SMILES | COC1=CC2=C(C=C1)C3=NC=CC4=C3C(=C(C(=C4OC)OC)O)C2=O | ||
| Standard InChIKey | HVXLZTVOIBKOPD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H15NO5/c1-23-9-4-5-10-12(8-9)16(21)14-13-11(6-7-20-15(10)13)18(24-2)19(25-3)17(14)22/h4-8,22H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dauriporphinoline is a natural product from Euchresta japonica. |
| In vitro | Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of Sinomenium acutum.[Pubmed: 16964757]Arch Pharm Res. 2006 Aug;29(8):627-32.Chromatographic separation of the MeOH extract from the stems and rhizomes of Sinomemium acutum led to the isolation of nine alkaloids and a lignan.
A sensitive and selective UPLC-MS/MS method for simultaneous determination of 10 alkaloids from Rhizoma Menispermi in rat plasma and its application to a pharmacokinetic study.[Pubmed: 26452875 ]Talanta. 2015 Nov 1;144:662-70.A sensitive and selective liquid chromatography-tandem mass spectrometry method has been developed and validated for simultaneous quantitation of 10 alkaloids (dauricine, daurisoline, N-desmethyldauricine, dauricicoline, Dauriporphinoline, bianfugecine, dauricoside, stepholidine, acutumine and acutumidine) from Rhizoma Menispermi in rat plasma.
|

Dauriporphinoline Dilution Calculator

Dauriporphinoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9645 mL | 14.8223 mL | 29.6446 mL | 59.2891 mL | 74.1114 mL |
| 5 mM | 0.5929 mL | 2.9645 mL | 5.9289 mL | 11.8578 mL | 14.8223 mL |
| 10 mM | 0.2964 mL | 1.4822 mL | 2.9645 mL | 5.9289 mL | 7.4111 mL |
| 50 mM | 0.0593 mL | 0.2964 mL | 0.5929 mL | 1.1858 mL | 1.4822 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2964 mL | 0.5929 mL | 0.7411 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anisole
Catalog No.:BCN2619
CAS No.:100-66-3
- Benzaldehyde
Catalog No.:BCN8529
CAS No.:100-52-7
- Benzylamine
Catalog No.:BCN1789
CAS No.:100-46-9
- Pentamidine
Catalog No.:BCC3836
CAS No.:100-33-4
- 4-Methoxybenzoic acid
Catalog No.:BCN3838
CAS No.:100-09-4
- 2-Methylthioadenosine triphosphate tetrasodium salt
Catalog No.:BCC6918
CAS No.:100020-57-3
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- GIP (human)
Catalog No.:BCC5870
CAS No.:100040-31-1
- TAK-875
Catalog No.:BCC3702
CAS No.:1000413-72-8
- 8-Hydroxy-3,5,6,7,3',4'-hexamethoxyflavone
Catalog No.:BCN7870
CAS No.:1000415-56-4
- AS 19
Catalog No.:BCC7218
CAS No.:1000578-26-6
- KW 2449
Catalog No.:BCC2179
CAS No.:1000669-72-6
- Stilbostemin N
Catalog No.:BCN4741
CAS No.:1000676-45-8
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- 7-Hydroxy-2',5,8-trimethoxyflavanone
Catalog No.:BCN5817
CAS No.:100079-34-3
- 2',4'-Dihydroxy-2,3',6'-trimethoxychalcone
Catalog No.:BCN1643
CAS No.:100079-39-8
- H-Lys(Tfa)-OH
Catalog No.:BCC2985
CAS No.:10009-20-8
Aporphine alkaloids and their reversal activity of multidrug resistance (MDR) from the stems and rhizomes of Sinomenium acutum.[Pubmed:16964757]
Arch Pharm Res. 2006 Aug;29(8):627-32.
Chromatographic separation of the MeOH extract from the stems and rhizomes of Sinomemium acutum led to the isolation of nine alkaloids and a lignan. Their structures were determined to be dauriporphine (1), bianfugecine (2), Dauriporphinoline (3), menisporphine (4), (-)-syringaresinol (5), N-feruloyltyramine (6), acutumine (7), dauricumine (8), sinomenine (9), and magnoflorine (10) by spectroscopic means. These compounds were examined for their P-gp mediated MDR reversal activity in human cancer cells. Compound 1 showed the most potent P-gp MDR inhibition activity with an ED50 value 0.03 microg/mL and 0.00010 microg/mL in the MES-SA/DX5 and HCT15 cells, respectively.
A sensitive and selective UPLC-MS/MS method for simultaneous determination of 10 alkaloids from Rhizoma Menispermi in rat plasma and its application to a pharmacokinetic study.[Pubmed:26452875]
Talanta. 2015 Nov 1;144:662-70.
A sensitive and selective liquid chromatography-tandem mass spectrometry method has been developed and validated for simultaneous quantitation of 10 alkaloids (dauricine, daurisoline, N-desmethyldauricine, dauricicoline, Dauriporphinoline, bianfugecine, dauricoside, stepholidine, acutumine and acutumidine) from Rhizoma Menispermi in rat plasma. After addition of internal standard (verapamil), plasma samples were pretreated by a single-step protein precipitation with acetonitrile. Chromatographic separation was performed on a Waters BEH C18 column with gradient elution using a mobile phase composed of acetonitrile and water (containing 0.1% formic acid) at a flow rate of 0.3 mL/min. The analytes were detected without interference in the multiple reaction monitoring (MRM) mode with positive electrospray ionization. The validated method exhibited good linearity over a wide concentration range (r>/=0.9914), and the lower limits of quantification were 0.01-5.0 ng/mL for all the analytes. The intra-day and inter-day precisions (RSD) at three different levels were both less than 13.4% and the accuracies (RE) ranged from -12.8% to 13.5%. The mean extraction recoveries of analytes and IS from rat plasma were all more than 77%. The validated method was successfully applied to a comparative pharmacokinetic study of 10 alkaloids in rat plasma after oral administration of Rhizoma Menispermi extract.


