BenzaldehydeCAS# 100-52-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100-52-7 | SDF | Download SDF |
| PubChem ID | 240 | Appearance | Colorless liquid |
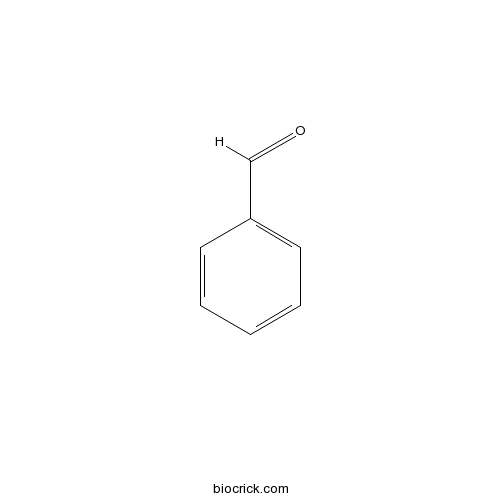
| Formula | C7H6O | M.Wt | 106.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Benzoic aldehyde;Phenylmethanal;Benzenecarbonal | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | benzaldehyde | ||
| SMILES | C1=CC=C(C=C1)C=O | ||
| Standard InChIKey | HUMNYLRZRPPJDN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6O/c8-6-7-4-2-1-3-5-7/h1-6H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzaldehyde Dilution Calculator

Benzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.4233 mL | 47.1165 mL | 94.2329 mL | 188.4659 mL | 235.5824 mL |
| 5 mM | 1.8847 mL | 9.4233 mL | 18.8466 mL | 37.6932 mL | 47.1165 mL |
| 10 mM | 0.9423 mL | 4.7116 mL | 9.4233 mL | 18.8466 mL | 23.5582 mL |
| 50 mM | 0.1885 mL | 0.9423 mL | 1.8847 mL | 3.7693 mL | 4.7116 mL |
| 100 mM | 0.0942 mL | 0.4712 mL | 0.9423 mL | 1.8847 mL | 2.3558 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Benzylamine
Catalog No.:BCN1789
CAS No.:100-46-9
- Pentamidine
Catalog No.:BCC3836
CAS No.:100-33-4
- 4-Methoxybenzoic acid
Catalog No.:BCN3838
CAS No.:100-09-4
- Anisole
Catalog No.:BCN2619
CAS No.:100-66-3
- Dauriporphinoline
Catalog No.:BCN7901
CAS No.:100009-82-3
- 2-Methylthioadenosine triphosphate tetrasodium salt
Catalog No.:BCC6918
CAS No.:100020-57-3
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- GIP (human)
Catalog No.:BCC5870
CAS No.:100040-31-1
- TAK-875
Catalog No.:BCC3702
CAS No.:1000413-72-8
- 8-Hydroxy-3,5,6,7,3',4'-hexamethoxyflavone
Catalog No.:BCN7870
CAS No.:1000415-56-4
- AS 19
Catalog No.:BCC7218
CAS No.:1000578-26-6
- KW 2449
Catalog No.:BCC2179
CAS No.:1000669-72-6
- Stilbostemin N
Catalog No.:BCN4741
CAS No.:1000676-45-8
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- 7-Hydroxy-2',5,8-trimethoxyflavanone
Catalog No.:BCN5817
CAS No.:100079-34-3
Photocatalytic degradation properties of alpha-Fe2O3 nanoparticles for dibutyl phthalate in aqueous solution system.[Pubmed:29765674]
R Soc Open Sci. 2018 Apr 11;5(4):172196.
The phthalate ester compounds in industrial wastewater, as kinds of environmental toxic organic pollutants, may interfere with the body's endocrine system, resulting in great harm to humans. In this work, the photocatalytic degradation properties of dibutyl phthalate (DBP) were investigated using alpha-Fe2O3 nanoparticles and H2O2 in aqueous solution system. The optimal parameters and mechanism of degradation were discussed by changing the morphology and usage amount of catalysts, the dosage of H2O2, pH value and the initial concentration of DBP. Hollow alpha-Fe2O3 nanoparticles showed the highest degradation efficiency when 30 mg of catalyst and 50 microl of H2O2 were used in the DBP solution with the initial concentration of 13 mg l(-1) at pH = 6.5. When the reaction time was 90 min, DBP was degraded 93% for the above optimal parameters. The photocatalytic degradation mechanism of DBP was studied by the gas chromatography-mass spectrometry technique. The result showed that the main degradation intermediates of DBP were ortho-phthalate monobutyl ester, methyl benzoic acid, benzoic acid, Benzaldehyde, and heptyl aldehyde when the reaction time was 2 h. DBP and its intermediates were almost completely degraded to CO2 and H2O in 12 h in the alpha-Fe2O3/ H2O2/UV system.
Crystal structures and anti-oxidant capacity of (E)-5-benz-yloxy-2-{[(4-chloro-phen-yl)imino]-meth-yl}phenol and (E)-5-benz-yloxy-2-({[2-(1H-indol-3-yl)eth-yl]iminium-yl}meth-yl)phenolate.[Pubmed:29765750]
Acta Crystallogr E Crystallogr Commun. 2018 Mar 9;74(Pt 4):478-482.
The title Schiff base compounds, C20H16ClNO2 (I) and C24H22N2O2 (II), were synthesized via the condensation reaction of 2-amino-4-chloro-phenol for (I), and 2-(2,3-di-hydro-1H-indol-3-yl)ethan-1-amine for (II), with 4-benz-yloxy-2-hy-droxy-Benzaldehyde. In both compounds, the configuration about the C=N imine bond is E. Neither mol-ecule is planar. In (I), the central benzene ring makes dihedral angles of 49.91 (12) and 53.52 (11) degrees with the outer phenyl and chloro-phenyl rings, respectively. In (II), the central benzene ring makes dihedral angles of 89.59 (9) and 72.27 (7) degrees , respectively, with the outer phenyl ring and the mean plane of the indole ring system (r.m.s. deviation = 0.011 A). In both compounds there is an intra-molecular hydrogen bond forming an S(6) ring motif; an O-Hcdots, three dots, centeredO hydrogen bond in (I), but a charge-assisted N(+)-Hcdots, three dots, centeredO(-) hydrogen bond in (II). In the crystal of (I), mol-ecules are linked by C-Hcdots, three dots, centeredpi inter-actions, forming slabs parallel to plane (001). In the crystal of (II), mol-ecules are linked by pairs of N-Hcdots, three dots, centeredO hydrogen bonds, forming inversion dimers. The dimers are linked by C-Hcdots, three dots, centeredO hydrogen bonds, C-Hcdots, three dots, centeredpi inter-actions and a weak N-Hcdots, three dots, centeredpi inter-action, forming columns propagating along the a-axis direction. The anti-oxidant capacity of the synthesized compounds was determined by cupric reducing anti-oxidant capacity (CUPRAC) for compound (I) and by 2,2-picrylhydrazyl hydrate (DPPH) for compound (II).
Benzaldehyde Synergizes the Response of Female Xyleborinus saxesenii (Coleoptera: Curculionidae, Scolytinae) to Ethanol.[Pubmed:29767753]
J Econ Entomol. 2018 May 15. pii: 4996095.
The ambrosia beetle, Xyleborinus saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae), infests physiologically stressed apple and peach trees in Korea. Dispersing females utilize the degradation product ethanol and host-related volatiles to locate and colonize new host trees. We examined the extent to which 12 chemicals emitted from fruit trees act synergistically with ethanol to attract X. saxesenii. The addition of Benzaldehyde to ethanol significantly increased beetle attraction, although Benzaldehyde was not attractive by itself. The addition of (-)-alpha-pinene, ethyl butyrate, ethyl isovalerate, (R)-(+)-limonene, 3-methyl-1-butanol, ethyl tiglate, (+)-aromadendrene, vanillin, 2-butanol, styrene, or ethyl 3,3-dimethylacrylate to ethanol had no effect on beetle attraction. In a dose-response test, the addition of 5-50% Benzaldehyde doses synergistically increased the number of beetle captures; however, trap catches did not increase as the Benzaldehyde dosage increased. The synergistic influence of Benzaldehyde on beetle response to ethanol was lower in early spring than in late summer to early fall, probably because synthetic Benzaldehyde emissions from field lures were overwhelmed by background levels of natural Benzaldehyde emitted from peach twigs in the flowering stage.
Crystal structure and Hirshfeld surface analysis of 7-eth-oxy-5-methyl-2-(pyridin-3-yl)-11,12-di-hydro-5,11-methano-[1,2,4]triazolo[1 ,5-c][1,3,5]benzoxadiazo-cine.[Pubmed:29765725]
Acta Crystallogr E Crystallogr Commun. 2018 Feb 20;74(Pt 3):367-370.
The title compound, C19H19N5O2, was prepared by the reaction of 3-amino-5-(pyridin-3-yl)-1,2,4-triazole with acetone and 2-hy-droxy-3-eth-oxy-Benzaldehyde. It crystallizes from ethanol in a tetra-gonal space group, with one mol-ecule in the asymmetric unit. The 1,2,4-triazole five-membered ring is planar (maximum deviation = 0.0028 A). The pyridine and phenyl rings are also planar with maximum deviations of 0.0091 and 0.0094 A, respectively. In the crystal, N-Hcdots, three dots, centeredN hydrogen bonds link the mol-ecules into supra-molecular chains propagating along the c-axis direction. Hirshfeld surface analysis and two-dimensional fingerprint plots have been used to analyse the inter-molecular inter-actions present in the crystal.
Crystal structure of N,N'-dibenzyl-3,3'-di-meth-oxy-benzidine.[Pubmed:29765704]
Acta Crystallogr E Crystallogr Commun. 2018 Feb 2;74(Pt 3):271-274.
The title compound, (systematic name: N,N'-dibenzyl-3,3'-dimeth-oxy-1,1'-biphenyl-4,4'-di-amine), C28H28N2O2, was synthesized by the reduction of a Schiff base prepared via a condensation reaction between o-dianisidine and Benzaldehyde under acidic conditions. The mol-ecule lies on a crystallographic inversion centre so that the asymmetric unit contains one half-mol-ecule. The biphenyl moiety compound is essentially planar. Two intra-molecular N-Hcdots, three dots, centeredO hydrogen bonds occur. The dihedral angle between the terminal phenyl and phenyl-ene rings of a benzidine unit is 48.68 (6) degrees . The methyl-ene C atom of the benzyl group is disordered over two sets of sites, with occupancy ratio 0.779 (18):0.221 (18). In the crystal, mol-ecules are connected by hydrogen bonding between o-dianisidine O atoms and H atoms of the terminal benzyl groups, forming a one-dimensional ladder-like structure. In the data from DFT calculations, the central biphenyl showed a twisted conformation.
The polystyrene-divinylbenzene stationary phase hybridized with oxidized nanodiamonds for liquid chromatography.[Pubmed:29759192]
Talanta. 2018 Aug 1;185:221-228.
A novel polystyrene-divinylbenzene microspheres hybridized with oxidized nanodiamonds (PS-DVB-OND) was synthesized by the method of seed swelling and polymerization. The oxidized nanodiamonds (OND) were characterized by Fourier transform infrared (FTIR) spectra, X-ray phtoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), X-ray diffraction (XRD). PS-DVB-OND particles were characterized by scanning electron microscopy (SEM) and transmission electron microscope (TEM). The result suggested that OND were successfully embedded into the polymer microspheres with the diameter of 6+/-2microm. Compared to polystyrene-divinylbenzene (PS-DVB) microspheres, PS-DVB-OND microspheres could tolerate higher pressure. The PS-DVB-OND microspheres were used as stationary phase of reversed-phase liquid chromatography directly and anion-exchangers after further quaternized with methylamine and 1,4-butanediol diglycidyl ether. Reversed-phase liquid chromatographic performance of PS-DVB-OND beads was investigated through separating six benzenes such as toluene, Benzaldehyde, phenol, benzoic acid, 1,4-hydroquinone and methyl p-hydroxybenzoate. Inorganic anions such as F(-), Cl(-), NO2(-), Br(-), NO3(-), HPO4(2-) and SO4(2-), were baseline separated on the anion exchangers of PS-DVB-OND microspheres. The result suggested that the prepared PS-DVB-OND microspheres have the potential as liquid chromatographic stationary phase under high pressure and extremely pH conditions.


