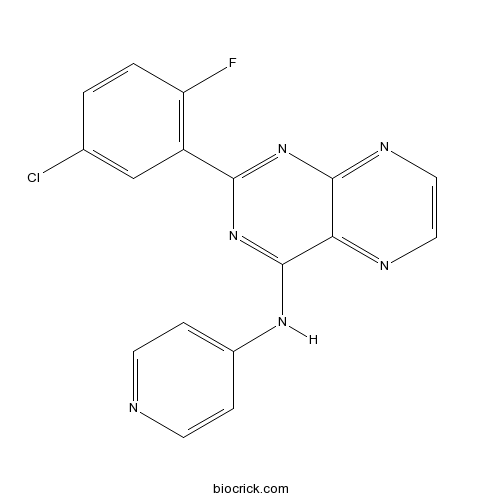
SD-208TGF-βR I kinase inhibitor CAS# 627536-09-8 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
- A 83-01
Catalog No.:BCC1319
CAS No.:909910-43-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 627536-09-8 | SDF | Download SDF |
| PubChem ID | 10316032 | Appearance | Powder |
| Formula | C17H10ClFN6 | M.Wt | 352.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 9.09 mg/mL (25.77 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2-(5-chloro-2-fluorophenyl)-N-pyridin-4-ylpteridin-4-amine | ||
| SMILES | C1=CC(=C(C=C1Cl)C2=NC3=NC=CN=C3C(=N2)NC4=CC=NC=C4)F | ||
| Standard InChIKey | BERLXWPRSBJFHO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H10ClFN6/c18-10-1-2-13(19)12(9-10)15-24-16-14(21-7-8-22-16)17(25-15)23-11-3-5-20-6-4-11/h1-9H,(H,20,22,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, orally active ATP-competitive transforming growth factor-β receptor 1 (TGF-βRI) inhibitor (IC50 = 49 nM). Displays > 100-fold and > 17-fold selectivity over TGF-βRII and other common kinases respectively. Exhibits anti-inflammatory and antitumor activity. Also promotes an antiglioma immune response. Ameliorates germinal matrix hemorrhage-induced neurological deficits in neonatal rats. |

SD-208 Dilution Calculator

SD-208 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8349 mL | 14.1743 mL | 28.3487 mL | 56.6974 mL | 70.8717 mL |
| 5 mM | 0.567 mL | 2.8349 mL | 5.6697 mL | 11.3395 mL | 14.1743 mL |
| 10 mM | 0.2835 mL | 1.4174 mL | 2.8349 mL | 5.6697 mL | 7.0872 mL |
| 50 mM | 0.0567 mL | 0.2835 mL | 0.567 mL | 1.1339 mL | 1.4174 mL |
| 100 mM | 0.0283 mL | 0.1417 mL | 0.2835 mL | 0.567 mL | 0.7087 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SD-208 Description:
EC50: SD-208 inhibits the growth inhibition of TGF-β–sensitive CCL64 cells at an EC50 of 0.1 μmol/L .
The cytokine transforming growth factor (TGF)-β has become a major target for the experimental treatment of human malignant gliomas. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human Glioma cells both in vitro and in vivo.
In vitro: SD-208 inhibits the growth inhibition of TGF-β–sensitive CCL64 cells mediated by recombinant TGF-β1 or TGF-β2 or of TGF-β–containing glioma cell supernatant at an EC50 of 0.1 μmol/L. SD-208 also blocks autocrine and paracrine TGF-β signaling in glioma cells as detected by the phosphorylation of Smad2 or TGF-β reporter assays and strongly inhibits constitutive and TGF-β–evoked migration and invasion, but not viability or proliferation. Moreover, SD-208 restores the lytic activity of polyclonal natural killer cells against glioma cells in the presence of recombinant TGF-β or of TGF-β–containing glioma cell supernatant. [1].
In vivo: The oral bioavailability of SD-208 was verified by demonstrating the inhibition of TGF-β–induced Smad phosphorylation in spleen and brain. Systemic SD-208 treatment initiated 3 days after the implantation of SMA-560 cells into the brains of syngeneic VM/Dk mice prolongs their median survival from 18.6 to 25.1 days. Histologic analysis revealed no difference in blood vessel formation, proliferation, or apoptosis. However, animals responding to SD-208 showed an increased tumor infiltration by natural killer cells, CD8 T cells, and macrophages. These data define TGF-β receptor I kinase inhibitors such as SD-208 as promising novel agents for the treatment of human malignant glioma and other conditions associated with pathological TGF-β activity [1].
Clinical trial: Up to now, SD-208 is still in the preclinical development stage.
Reference:
[1] Leung SY, Niimi A, Noble A, Oates T, Williams AS, Medicherla S, Protter AA, Chung KF. Effect of transforming growth factor-beta receptor I kinase inhibitor 2,4-disubstituted pteridine (SD-208) in chronic allergic airway inflammation and remodeling. J Pharmacol Exp Ther. 2006;319(2):586-94.
- BMS-564929
Catalog No.:BCC1425
CAS No.:627530-84-1
- 4-Amino-N-methylbenzamide
Catalog No.:BCC8685
CAS No.:6274-22-2
- SKF38393 HCl
Catalog No.:BCC6526
CAS No.:62717-42-4
- Dihydrolycorine
Catalog No.:BCN2475
CAS No.:6271-21-2
- NF 449
Catalog No.:BCC7043
CAS No.:627034-85-9
- Dioctanoylglycol
Catalog No.:BCC6662
CAS No.:627-86-1
- H-D-Arg-OH.HCl
Catalog No.:BCC2869
CAS No.:627-75-8
- Saikosaponin F
Catalog No.:BCN2776
CAS No.:62687-63-2
- Handelin
Catalog No.:BCN2953
CAS No.:62687-22-3
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- Epitrametol
Catalog No.:BCN7073
CAS No.:627538-65-2
- Pterophorine
Catalog No.:BCN2118
CAS No.:62786-99-6
- Senampeline A
Catalog No.:BCN2030
CAS No.:62787-00-2
- Senampeline D
Catalog No.:BCN2033
CAS No.:62787-01-3
- SU14813
Catalog No.:BCC1971
CAS No.:627908-92-3
- Palmitic acid ethyl ester
Catalog No.:BCN8298
CAS No.:628-97-7
- Jolkinol A
Catalog No.:BCN3778
CAS No.:62820-11-5
- Meglumine
Catalog No.:BCC4795
CAS No.:6284-40-8
- Senampeline E
Catalog No.:BCN2032
CAS No.:71075-42-8
- 2-Amino-6-nitrobenzothiazole
Catalog No.:BCC8544
CAS No.:6285-57-0
- Senampeline B
Catalog No.:BCN2031
CAS No.:62860-52-0
- TP 003
Catalog No.:BCC6169
CAS No.:628690-75-5
Down-regulation of miR-135b in colon adenocarcinoma induced by a TGF-beta receptor I kinase inhibitor (SD-208).[Pubmed:26523217]
Iran J Basic Med Sci. 2015 Sep;18(9):856-61.
OBJECTIVES: Transforming growth factor-beta (TGF-beta) is involved in colorectal cancer (CRC). The SD-208 acts as an anti-cancer agent in different malignancies via TGF-beta signaling. This work aims to show the effect of manipulation of TGF-beta signaling on some miRNAs implicated in CRC. MATERIALS AND METHODS: We investigated the effects of SD-208 on SW-48, a colon adenocarcinoma cell line. The cell line was treated with 0.5, 1 and 2 muM concentrations of SD-208. Then, the xenograft model of colon cancer was established by subcutaneous inoculation of SW-48 cell line into the nude mice. The animals were treated with SD-208 for three weeks. A quantitative real-time PCR was carried out for expression level analysis of selected oncogenic (miR-21, 31, 20a and 135b) and suppressor-miRNAs (let7-g, miR-133b, 145 and 200c). Data were analyzed using the 2-CT method through student's t-test via the GraphPad Prism software. RESULTS: Our results revealed that SD-208 could significantly down-regulate the expression of one key onco-miRNA, miR-135b, in either SW-48 colon cells (P=0.006) or tumors orthotopically implanted in nude mice (P=0.018). Our in silico study also predicted that SD-208 could modulate the expression of potential downstream tumor suppressor targets of the miR135b. CONCLUSION: Our data provide novel evidence that anticancer effects of SD-208 (and likely other TGF-beta inhibitors) may be owing to their ability to regulate miRNAs expression.
[Effects of transforming growth factor beta1 receptor inhibitor SD-208 on human hypertrophic scar].[Pubmed:27464628]
Zhonghua Shao Shang Za Zhi. 2016 Jul 20;32(7):389-95.
OBJECTIVE: To investigate the effects of transforming growth factor beta1 (TGF-beta1) receptor inhibitor SD-208 on human hypertrophic scar and its mechanisms. METHODS: Scar fibroblasts were isolated from deprecated human hypertrophic scar tissue and then sub-cultured. Cells of the fifth passage were used in the following experiments. (1) Cells were divided into blank control group (BC) and 0.5, 1.0, 3.0, and 5.0 mumol/L SD-208 groups according to the random number table (the same grouping method below), with 6 wells in each group. Cells in group BC were added with 1 muL phosphate buffer solution, while cells in the latter four groups were added with 0.5, 1.0, 3.0, and 5.0 mumol/L SD-208, respectively. After being cultured for 12 hours, the proliferation activity of cells was detected by cell counting kit 8 and microplate reader (denoted as absorbance value). Suitable amount of substance concentration of SD-208 according to the results of proliferation activity of cells was chosen for the following experiments. (2) Another batch of cells were divided into group BC and 1, 3 mumol/L SD-208 groups and treated as in (1), with 8 wells in each group. The number of migration cells was detected by transwell method. (3) Another batch of cells were grouped and treated as in (2), and the microfilament morphology of cells was observed by rhodamine-phalloidin staining. (4) Another batch of cells were grouped and treated as in (2), and the protein expression of TGF-beta1 was assessed with Western blotting. (5) Forty-eight BALB/c nude mice were divided into normal saline group (NS) and 1 mumol/L SD-208 group, and one longitudinal incision with length of 1 cm was made on their back. Then human hypertrophic scar tissue was embedded into the incision. On post injury day 7, multipoint injection of NS in a volume of 0.05 mL was performed in wounds of rats in group NS, while rats in 1 mumol/L SD-208 group were given 0.05 mL 1 mumol/L SD-208, once a day. On the day 0 (the same day), 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 post first time of injection, the weight of 8 nude mice was weighed by electronic scale, and scar area was measured by vernier caliper and the ratio of rest scar area was calculated. (6) In week 1, 2, and 3 post first time of injection, the protein expression of TGF-beta1 of human hypertrophic scar tissue was assessed with Western blotting. Data were processed with one-way analysis of variance and two independent-sample t test. RESULTS: (1) The proliferation activity of cells in group BC, 0.5, 1.0, 3.0, and 5.0 mumol/L SD-208 groups was respectively 1.00+/-0.03, 0.90+/-0.08, 0.68+/-0.11, 0.54+/-0.04, and 0.42+/-0.09, and the proliferation activity of cells in 0.5, 1.0, 3.0, and 5.0 mumol/L SD-208 groups was significantly lower than that in group BC (with t values from 2.9 to 22.1, P<0.05 or P<0.01). (2) The number of migration cells in 1, 3 mumol/L SD-208 groups was significantly less than that in group BC (with t values respectively 6.5 and 6.4, P values below 0.01). (3) Compared with that in group BC, fluorescence intensity of microfilaments of cells in 1, 3 mumol/L SD-208 groups was attenuated, and the pseudopod extended less. (4) The protein expressions of TGF-beta1 of cells in group BC and 1, 3 mumol/L SD-208 groups were respectively 1.00+/-0.08, 0.80+/-0.08, and 0.61+/-0.05, and the protein expressions of TGF-beta1 of cells in 1, 3 mumol/L SD-208 groups were significantly lower than those in group BC (with t values respectively 4.0 and 9.2, P values below 0.01). (5) The weights of nude mice in group NS and 1 mumol/L SD-208 group were similar on each time day (with t values from 0.2 to 1.1, P values above 0.05). The ratios of rest scar area of nude mice in two groups were decreased along with the injection time, and the ratios of rest scar area of nude mice in 1 mumol/L SD-208 group were significantly less than those in group NS from the day 6 to 20 post first time of injection (with t values from 1.8 to 15.9, P<0.05 or P<0.01). In week 1, 2, and 3 post first time of injection, the protein expressions of TGF-beta1 of human hypertrophic scar tissue in nude mice in two groups showed a tendency of decrease, and the protein expressions of TGF-beta1 of human hypertrophic scar tissue in nude mice in 1 mumol/L SD-208 group were significantly lower than those in group NS (with t values from 6.2 to 19.1, P values below 0.01). CONCLUSIONS: SD-208 has significant inhibition effect on human hypertrophic scars, and the mechanism is correlated to the inhibition of protein expression of endogenous TGF-beta1.
Evaluation of SD-208, a TGF-beta-RI Kinase Inhibitor, as an Anticancer Agent in Retinoblastoma.[Pubmed:27306340]
Acta Med Iran. 2016 Jun;54(6):352-8.
Retinoblastoma is the most common intraocular tumor in children resulting from genetic alterations and transformation of mature retinal cells. The objective of this study was to investigate the effects of SD-208, TGF-beta-RI kinase inhibitor, on the expression of some miRNAs including a miR-17/92 cluster in retinoblastoma cells. Prior to initiate this work, the cell proliferation was studied by Methyl Thiazolyl Tetrazolium (MTT) and bromo-2'-deoxyuridine (BrdU) assays. Then, the expression patterns of four miRNAs (18a, 20a, 22, and 34a) were investigated in the treated SD-208 (0.0, 1, 2 and 3 microM) and untreated Y-79 cells. A remarkable inhibition of the cell proliferation was found in Y-79 cells treated with SD-208 versus untreated cells. Also, the expression changes were observed in miRNAs 18a, 20a, 22 and 34a in response to SD-208 treatment (P<0.05). The findings of the present study suggest that the anti-cancer effect of SD-208 may be exerted due to the regulation of specific miRNAs, at least in this particular retinoblastoma cell line. To the best of the researchers' knowledge, this is the first report demonstrating that the SD-208 could alter the expression of tumor suppressive miRNAs as well as oncomiRs in vitro. In conclusion, the present data suggest that SD-208 could be an alternative agent in retinoblastoma treatment.
SD-208, a novel protein kinase D inhibitor, blocks prostate cancer cell proliferation and tumor growth in vivo by inducing G2/M cell cycle arrest.[Pubmed:25747583]
PLoS One. 2015 Mar 6;10(3):e0119346.
Protein kinase D (PKD) has been implicated in many aspects of tumorigenesis and progression, and is an emerging molecular target for the development of anticancer therapy. Despite recent advancement in the development of potent and selective PKD small molecule inhibitors, the availability of in vivo active PKD inhibitors remains sparse. In this study, we describe the discovery of a novel PKD small molecule inhibitor, SD-208, from a targeted kinase inhibitor library screen, and the synthesis of a series of analogs to probe the structure-activity relationship (SAR) vs. PKD1. SD-208 displayed a narrow SAR profile, was an ATP-competitive pan-PKD inhibitor with low nanomolar potency and was cell active. Targeted inhibition of PKD by SD-208 resulted in potent inhibition of cell proliferation, an effect that could be reversed by overexpressed PKD1 or PKD3. SD-208 also blocked prostate cancer cell survival and invasion, and arrested cells in the G2/M phase of the cell cycle. Mechanistically, SD-208-induced G2/M arrest was accompanied by an increase in levels of p21 in DU145 and PC3 cells as well as elevated phosphorylation of Cdc2 and Cdc25C in DU145 cells. Most importantly, SD-208 given orally for 24 days significantly abrogated the growth of PC3 subcutaneous tumor xenografts in nude mice, which was accompanied by reduced proliferation and increased apoptosis and decreased expression of PKD biomarkers including survivin and Bcl-xL. Our study has identified SD-208 as a novel efficacious PKD small molecule inhibitor, demonstrating the therapeutic potential of targeted inhibition of PKD for prostate cancer treatment.
Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage.[Pubmed:24425124]
Stroke. 2014 Mar;45(3):828-34.
BACKGROUND AND PURPOSE: Transforming growth factor-beta (TGF-beta) overproduction and activation of the TGF-beta pathway are associated with the development of brain injury following germinal matrix hemorrhage (GMH) in premature infants. We examined the effects of GMH on the level of TGF-beta1 in a novel rat collagenase-induced GMH model and determined the effect of inhibition of the TGF receptor I. METHODS: In total, 92 seven-day old (P7) rats were used. Time-dependent effects of GMH on the level of TGF-beta1 and TGF receptor I were evaluated by Western blot. A TGF receptor I inhibitor (SD208) was administered daily for 3 days, starting either 1 hour or 3 days after GMH induction. The effects of GMH and SD208 on the TGF-beta pathway were evaluated by Western blot at day 3. The effects of GMH and SD208 on cognitive and motor function were also assessed. The effects of TGF receptor I inhibition by SD208 on GMH-induced brain injury and underlying molecular pathways were investigated by Western blot, immunofluorescence, and morphology studies 24 days after GMH. RESULTS: GMH induced significant delay in development, caused impairment in both cognitive and motor functions, and resulted in brain atrophy in rat subjects. GMH also caused deposition of both vitronectin (an extracellular matrix protein) and glial fibrillary acidic protein in perilesion areas, associated with development of hydrocephalus. SD208 ameliorated GMH-induced developmental delay, improved cognitive and motor functions, and attenuated body weight loss. SD208 also decreased vitronectin and glial fibrillary acidic protein deposition and decreased GMH-induced brain injury. CONCLUSIONS: Increased level of TGF-beta1 and activation of the TGF-beta pathway associate with the development of brain injury after GMH. SD208 inhibits GMH-induced activation of the TGF-beta pathway and leads to an improved developmental profile, partial recovery of cognitive and motor functions, and attenuation of GMH-induced brain atrophy and hydrocephalus.
Transforming growth factor-beta receptor type 1 (TGFbetaRI) kinase activity but not p38 activation is required for TGFbetaRI-induced myofibroblast differentiation and profibrotic gene expression.[Pubmed:16707625]
Mol Pharmacol. 2006 Aug;70(2):518-31.
Transforming growth factor-beta (TGFbeta) is a major mediator of normal wound healing and of pathological conditions involving fibrosis, such as idiopathic pulmonary fibrosis. TGFbeta also stimulates the differentiation of myofibroblasts, a hallmark of fibrotic diseases. In this study, we examined the underlying processes of TGFbetaRI kinase activity in myofibroblast conversion of human lung fibroblasts using specific inhibitors of TGFbetaRI (SD-208) and p38 mitogen-activated kinase (SD-282). We demonstrated that SD-208, but not SD-282, inhibited TGFbeta-induced SMAD signaling, myofibroblast transformation, and collagen gel contraction. Furthermore, we extended our findings to a rat bleomycin-induced lung fibrosis model, demonstrating a significant decrease in the number of myofibroblasts at fibroblastic foci in animals treated with SD-208 but not those treated with SD-282. SD-208 also reduced collagen deposition in this in vivo model. Microarray analysis of human lung fibroblasts identified molecular fingerprints of these processes and showed that SD-208 had global effects on reversing TGFbeta-induced genes involved in fibrosis, inflammation, cell proliferation, cytoskeletal organization, and apoptosis. These studies also revealed that although the p38 pathway may not be needed for appearance or disappearance of the myofibroblast, it can mediate a subset of inflammatory and fibrogenic events of the myofibroblast during the process of tissue repair and fibrosis. Our findings suggest that inhibitors such as SD-208 may be therapeutically useful in human interstitial lung diseases and pulmonary fibrosis.
Effect of transforming growth factor-beta receptor I kinase inhibitor 2,4-disubstituted pteridine (SD-208) in chronic allergic airway inflammation and remodeling.[Pubmed:16888081]
J Pharmacol Exp Ther. 2006 Nov;319(2):586-94.
Transforming growth factor (TGF)-beta is a multifunctional regulator of cell growth and differentiation with both pro- and anti-inflammatory properties. We used an inhibitor of TGF-beta receptor I (TGF-betaRI) kinase, SD-208 (2,4-disubstituted pteridine, a ATP-competitive inhibitor of TGF-betaRI kinase), to determine the role of TGF-beta in airway allergic inflammation and remodeling. Brown-Norway rats sensitized and repeatedly exposed to ovalbumin (OVA) aerosol challenge were orally administered SD-208 twice daily, before each of six OVA exposures to determine the preventive effects, or only before each of the last three of six OVA exposures to investigate its reversal effects. SD-208 (60 mg/kg) reversed bronchial hyperresponsiveness (BHR) induced by repeated allergen exposure, but it did not prevent it. SD-208 prevented changes in serum total and OVA-specific IgE, but it did not reverse them. SD-208 had both a preventive and reversal effect on airway inflammation as measured by major basic protein-positive eosinophils and CD2(+) T-cell counts in mucosal airways, cell proliferation measured by 5-bromo-2'-deoxyuridine expression in airway smooth muscle (ASM) cells and epithelial cells, and goblet cell hyperplasia induced by repeated allergen challenges. There was a significant decrease in intracellular Smad2/3 expression. SD-208 did not significantly decrease the increased ASM thickness induced by allergen exposure. These findings support a proinflammatory and proremodeling role for TGF-beta in allergic airway inflammation. Inhibition of TGF-betaRI kinase activities by SD-208 may be a useful approach to the reversal of BHR and to the prevention and reversal of inflammatory and remodeling features of chronic asthma.
SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo.[Pubmed:15520202]
Cancer Res. 2004 Nov 1;64(21):7954-61.
The cytokine transforming growth factor (TGF)-beta, by virtue of its immunosuppressive and promigratory properties, has become a major target for the experimental treatment of human malignant gliomas. Here we characterize the effects of a novel TGF-beta receptor (TGF-betaR) I kinase inhibitor, SD-208, on the growth and immunogenicity of murine SMA-560 and human LN-308 glioma cells in vitro and the growth of and immune response to intracranial SMA-560 gliomas in syngeneic VM/Dk mice in vivo. SD-208 inhibits the growth inhibition of TGF-beta-sensitive CCL64 cells mediated by recombinant TGF-beta1 or TGF-beta2 or of TGF-beta-containing glioma cell supernatant at an EC(50) of 0.1 mumol/L. SD-208 blocks autocrine and paracrine TGF-beta signaling in glioma cells as detected by the phosphorylation of Smad2 or TGF-beta reporter assays and strongly inhibits constitutive and TGF-beta-evoked migration and invasion, but not viability or proliferation. Peripheral blood lymphocytes or purified T cells, cocultured with TGF-beta-releasing LN-308 glioma cells in the presence of SD-208, exhibit enhanced lytic activity against LN-308 targets. The release of interferon gamma and tumor necrosis factor alpha by these immune effector cells is enhanced by SD-208, whereas the release of interleukin 10 is reduced. SD-208 restores the lytic activity of polyclonal natural killer cells against glioma cells in the presence of recombinant TGF-beta or of TGF-beta-containing glioma cell supernatant. The oral bioavailability of SD-208 was verified by demonstrating the inhibition of TGF-beta-induced Smad phosphorylation in spleen and brain. Systemic SD-208 treatment initiated 3 days after the implantation of SMA-560 cells into the brains of syngeneic VM/Dk mice prolongs their median survival from 18.6 to 25.1 days. Histologic analysis revealed no difference in blood vessel formation, proliferation, or apoptosis. However, animals responding to SD-208 showed an increased tumor infiltration by natural killer cells, CD8 T cells, and macrophages. These data define TGF-beta receptor I kinase inhibitors such as SD-208 as promising novel agents for the treatment of human malignant glioma and other conditions associated with pathological TGF-beta activity.


