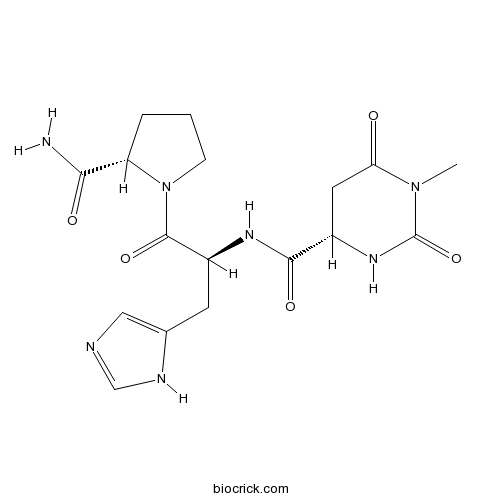
TaltirelinSynthetic thyrotropin-releasing hormone analog CAS# 103300-74-9 |
- SB-222200
Catalog No.:BCC1926
CAS No.:174635-69-9
- Talnetant hydrochloride
Catalog No.:BCC1982
CAS No.:204519-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103300-74-9 | SDF | Download SDF |
| PubChem ID | 114750 | Appearance | Powder |
| Formula | C17H23N7O5 | M.Wt | 405.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TA 0910 | ||
| Solubility | DMSO : ≥ 32 mg/mL (78.93 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (4S)-N-[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-1-methyl-2,6-dioxo-1,3-diazinane-4-carboxamide | ||
| SMILES | CN1C(=O)CC(NC1=O)C(=O)NC(CC2=CN=CN2)C(=O)N3CCCC3C(=O)N | ||
| Standard InChIKey | LQZAIAZUDWIVPM-SRVKXCTJSA-N | ||
| Standard InChI | InChI=1S/C17H23N7O5/c1-23-13(25)6-10(22-17(23)29)15(27)21-11(5-9-7-19-8-20-9)16(28)24-4-2-3-12(24)14(18)26/h7-8,10-12H,2-6H2,1H3,(H2,18,26)(H,19,20)(H,21,27)(H,22,29)/t10-,11-,12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Synthetic thyrotropin-releasing hormone (TRH) analog. Displays ~ 30 - 100-fold more potent CNS activity and ~ 50-fold weaker endocrine activity than TRH. Antinociceptive and neuroprotective. |

Taltirelin Dilution Calculator

Taltirelin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4666 mL | 12.3332 mL | 24.6664 mL | 49.3328 mL | 61.666 mL |
| 5 mM | 0.4933 mL | 2.4666 mL | 4.9333 mL | 9.8666 mL | 12.3332 mL |
| 10 mM | 0.2467 mL | 1.2333 mL | 2.4666 mL | 4.9333 mL | 6.1666 mL |
| 50 mM | 0.0493 mL | 0.2467 mL | 0.4933 mL | 0.9867 mL | 1.2333 mL |
| 100 mM | 0.0247 mL | 0.1233 mL | 0.2467 mL | 0.4933 mL | 0.6167 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
novel orally active TRH analogue, binds to rat brain TRH receptors in vivo.
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- PTIQ
Catalog No.:BCC7953
CAS No.:1032822-42-6
- GDC-0980 (RG7422)
Catalog No.:BCC4992
CAS No.:1032754-93-0
- GNE-477
Catalog No.:BCC8049
CAS No.:1032754-81-6
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- A939572
Catalog No.:BCC5305
CAS No.:1032229-33-6
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
- HPGDS inhibitor 1
Catalog No.:BCC4065
CAS No.:1033836-12-2
- 1-O-Methylnataloe-emodin
Catalog No.:BCN7036
CAS No.:103392-51-4
- Octyl gallate
Catalog No.:BCN8432
CAS No.:1034-01-1
Taltirelin, a thyrotropin-releasing hormone analog, alleviates mechanical allodynia through activation of descending monoaminergic neurons in persistent inflammatory pain.[Pubmed:21872219]
Brain Res. 2011 Sep 26;1414:50-7.
Thyrotropin-releasing hormone (TRH) and its analogs have been reported to modulate descending monoaminergic inhibitory neurons, resulting in antinociception. However, it remains unknown whether TRH exerts an antiallodynic effect during persistent pain. Here, we investigated the action of Taltirelin, a stable TRH analog, on mechanical allodynia in mice with inflammatory persistent pain induced by an injection of complete Freund's adjuvant into the hindpaw. Systemic administration of 1.0 mg/kg Taltirelin markedly reduced mechanical allodynia. This effect was abolished by the 6-hydroxydopamine (6-OHDA)-induced depletion of central noradrenaline. While intraperitoneal injection of the alpha(1)-adrenoceptor antagonist prazosin had no effect, intraperitoneal and intrathecal administration of the alpha(2)-adrenoceptor antagonist yohimbine prevented the antiallodynic action of Taltirelin. In addition, DL-p-chlorophenylalanine (PCPA)-induced depletion of serotonin (5-HT) and intraperitoneal and intrathecal injection of the 5-HT(1A) receptor antagonist WAY-100635 blocked the effect of Taltirelin on allodynia. These findings suggest that Taltirelin alleviates mechanical allodynia in inflammatory persistent pain by modulating the descending noradrenergic and serotonergic neuronal pathways via indirect activation of spinal alpha(2)-adrenergic and 5-HT(1A) receptors.
Reversal of hemorrhagic shock in rats using the metabolically stable thyrotropin-releasing hormone analog taltirelin hydrate.[Pubmed:22044177]
J Recept Signal Transduct Res. 2011 Dec;31(6):416-22.
We investigated the effect of Taltirelin hydrate ((-)-N-[(S)-hexahydro-1-methyl- 2,6-dioxo-4-pyrimidinyl-carbonyl]-L-histidyl-L-prolinamide tetrahydrate; Taltirelin), a metabolically stable thyrotropin-releasing hormone (TRH) analog, on circulatory function, respiratory function, and viable time after bleeding in urethane-anesthetized rats. Massive volume-controlled bleeding caused marked reductions in mean arterial pressure (MAP) and respiratory rate (RR). The vital signs of control rats were lost within an average of 23 min after bleeding. Intravenous administration of Taltirelin (0.03-0.3 mg/kg) and TRH (1 and 3 mg/kg) immediately after bleeding accelerated recovery of MAP and RR, and prolonged viable time in a dose-dependent manner. The potency of Taltirelin in accelerating MAP and RR recovery and prolonging viable time was higher when compared with that of TRH. In addition, recovery of MAP and RR and the extension of viable time by Taltirelin were inhibited by preintraperitoneal administration of atropine sulfate, which is a centrally acting muscarinic antagonist, but not by that of atropine methylbromide, which is a peripherally acting muscarinic antagonist. Taltirelin also recovered decreased arterial pH, bicarbonate ions, and base excess, and prevented a decrease in arterial oxygen saturation. In conclusion, the anti-shock effect of Taltirelin was more potent than that of TRH. Taltirelin activity was mediated by the central muscarinic cholinergic system. In addition, Taltirelin also corrected metabolic acidosis. These results suggest that Taltirelin could be useful in the treatment of hypovolemic shock.
Taltirelin is a superagonist at the human thyrotropin-releasing hormone receptor.[Pubmed:23087672]
Front Endocrinol (Lausanne). 2012 Oct 9;3:120.
Taltirelin (TAL) is a thyrotropin-releasing hormone (TRH) analog that is approved for use in humans in Japan. In this study, we characterized TAL binding to and signaling by the human TRH receptor (TRH-R) in a model cell system. We found that TAL exhibited lower binding affinities than TRH and lower signaling potency via the inositol-1,4,5-trisphosphate/calcium pathway than TRH. However, TAL exhibited higher intrinsic efficacy than TRH in stimulating inositol-1,4,5-trisphosphate second messenger generation. This is the first study that elucidates the pharmacology of TAL at TRH-R and shows that TAL is a superagonist at TRH-R. We suggest the superagonism exhibited by TAL may in part explain its higher activity in mediating central nervous system effects in humans compared to TRH.
Thyrotropin-releasing hormone receptor type 1 (TRH-R1), not TRH-R2, primarily mediates taltirelin actions in the CNS of mice.[Pubmed:23303050]
Neuropsychopharmacology. 2013 May;38(6):950-6.
Thyrotropin-releasing hormone receptor type 2 (TRH-R2), not TRH-R1, has been proposed to mediate the CNS effects of TRH and its more effective analog Taltirelin (TAL). Consistent with this idea, TAL exhibited higher binding affinity and signaling potency at mouse TRH-R2 than TRH-R1 in a model cell system. We used TRH-R1 knockout (R1ko), R2ko and R1/R2ko mice to determine which receptor mediates the CNS effects of TAL. There was no TRH-R1 mRNA in R1ko and R1/R2ko mice and no TRH-R2 mRNA in R2ko and R1/R2ko mice. Specific [(3)H]MeTRH binding to whole brain membranes was 5% of wild type (WT) for R1ko mice, 100% for R2ko mice and 0% for R1/R2ko mice, indicating TRH-R1 is the predominant receptor expressed in the brain. In arousal assays, TAL shortened sleep time with pentobarbital sedation in WT and R2ko mice by 44 and 49% and with ketamine/xylazine sedation by 66 and 55%, but had no effect in R1ko and R1/R2ko mice. In a tail flick assay of nociception, TAL increased response latency by 65 and 70% in WT and R2ko mice, but had no effect in R1ko and R1/R2ko mice. In a tail suspension test of depression-like behavior, TAL increased mobility time by 49 and 37% in WT and R2ko mice, but had no effect in R1ko and R1/R2ko mice. Thus, in contrast to the generally accepted view that the CNS effects of TAL are mediated by TRH-R2, these effects are mediated primarily if not exclusively by TRH-R1 in mice.
The synthetic TRH analogue taltirelin exerts modality-specific antinociceptive effects via distinct descending monoaminergic systems.[Pubmed:17220907]
Br J Pharmacol. 2007 Feb;150(4):403-14.
BACKGROUND AND PURPOSE: Exogenously administered thyrotropin-releasing hormone (TRH) is known to exert potent but short-acting centrally-mediated antinociceptive effects. We sought to investigate the mechanisms underlying these effects using the synthetic TRH analogue Taltirelin, focusing on the descending monoaminergic systems in mice. EXPERIMENTAL APPROACH: The mice received systemic or local injections of Taltirelin combined with either central noradrenaline (NA) or 5-hydroxytryptamine (5-HT) depletion by 6-hydroxydopamine (6-OHDA) or DL-p-chlorophenylalanine (PCPA), respectively, or blockade of their receptors. The degree of antinociception was determined using the tail flick and tail pressure tests. KEY RESULTS: Subcutaneously (s.c.) administered Taltirelin exhibited dose-dependent antinociceptive effects in the tail flick and tail pressure tests. These effects appeared to be primarily supraspinally mediated, since intracerebroventricularly (i.c.v.) but not intrathecally (i.t.) injected Taltirelin generated similar effects. Depletion of central NA abolished only the analgesic effect of Taltirelin (s.c. and i.c.v.) on mechanical nociception. By contrast, depletion of central 5-HT abolished only its analgesic effect on thermal nociception. Intraperitoneal (i.p.) and i.t. injection of the alpha2-adrenoceptor antagonist yohimbine respectively reduced the analgesic effect of Taltirelin (s.c. and i.c.v.) on mechanical nociception. By contrast, the 5-HT1A receptor antagonist WAY-100635 (i.p. and i.t.) reduced the effect of Taltirelin (s.c. and i.c.v.) on thermal nociception. Neither the 5-HT2 receptor antagonist ketanserin nor the opioid receptor antagonist naloxone altered the antinociceptive effect of Taltirelin. CONCLUSIONS AND IMPLICATIONS: These findings suggest that Taltirelin activates the descending noradrenergic and serotonergic pain inhibitory systems, respectively, to exert its analgesic effects on mechanical and thermal nociception.
Neuroprotective effect and brain receptor binding of taltirelin, a novel thyrotropin-releasing hormone (TRH) analogue, in transient forebrain ischemia of C57BL/6J mice.[Pubmed:12467901]
Life Sci. 2002 Dec 20;72(4-5):601-7.
Thyrotropin-releasing hormone (TRH) and some of its stable analogues have been shown to improve neurologic dysfunctions such as brain trauma in both animals and humans. Our previous study revealed that Taltirelin, a novel orally active TRH analogue, binds to rat brain TRH receptors in vivo. The present study was undertaken to investigate whether Taltirelin has neuroprotective effects in transient brain ischemia of C57BL/6J mice induced by bilateral carotid artery occlusion (2VO). Neuronal cell density in the hippocampal CA1 region of C57BL/6J mice was significantly (39.9%) decreased 1 week after 2VO-reperfusion, compared to the case of the sham group, and this reduction of hippocampal neuronal density was significantly suppressed by an intravenous (i.v.) injection of Taltirelin (0.3 mg/kg). The i.v. injection of Taltirelin at this dosage produced a significant increase in the dissociation constant (Kd) of specific [3H]MeTRH binding in sham and 2VO-reperfusion groups (33.6 and 51.4%, respectively) compared with the vehicle-treated group. These results indicate that the intravenously injected Taltirelin bound to TRH receptors in the ischemic brain. There was little difference in the brain-to-plasma concentration ratio (Kp) of [14C]sucrose between the sham and 2VO groups of C57BL/6J mice, indicating that the tight junction of the blood-brain barrier may be intact in the ischemic brain. In conclusion, the study has shown that Taltirelin may have a significant neuroprotective effect on the ischemic brain.
Synthesis and central nervous system actions of thyrotropin-releasing hormone analogues containing a dihydroorotic acid moiety.[Pubmed:2115588]
J Med Chem. 1990 Aug;33(8):2130-7.
A series of thyrotropin-releasing hormone (TRH) analogues in which the pyroglutamic acid residue was replaced by (S)-4,5-dihydroorotic acid (Dio-OH) and the related derivatives were prepared. Their central nervous system actions based on spontaneous locomotor activity, antagonistic effect on reserpine-induced hypothermia, and antagonistic effect on pentobarbital anesthesia were evaluated and the structure-activity relationships are discussed. Of these, (1-methyl-(S)-4,5-dihydroorotyl)-L-histidyl-L-prolinamide (14b) showed the most potent activities, which were 30-90 times greater than those of TRH. Moreover, the thyrotropin-releasing activity of 14b was about 50 times weaker than that of TRH, and compound 14b (TA-0910) was selected as a potent candidate.


