PTIQPotent MMP-3 expression inhibitor; neuroprotective CAS# 1032822-42-6 |
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- CFTRinh-172
Catalog No.:BCC4419
CAS No.:307510-92-5
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1032822-42-6 | SDF | Download SDF |
| PubChem ID | 24812799 | Appearance | Powder |
| Formula | C13H17NO3 | M.Wt | 235.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in 1eq. NaOH | ||
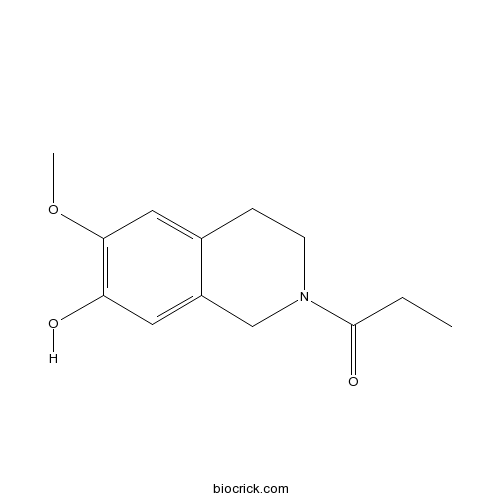
| Chemical Name | 1-(7-hydroxy-6-methoxy-3,4-dihydro-1H-isoquinolin-2-yl)propan-1-one | ||
| SMILES | CCC(=O)N1CCC2=CC(=C(C=C2C1)O)OC | ||
| Standard InChIKey | BEDYMEDICHGLSE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H17NO3/c1-3-13(16)14-5-4-9-7-12(17-2)11(15)6-10(9)8-14/h6-7,15H,3-5,8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of MMP-3 expression (IC50 = 60 nM); down-regulates induction of MMP-3 in both stressed dopaminergic cells and activated microglia. Also suppresses proinflammatory responses and inhibits NO production in activated microglia. Attenuates motor deficits, prevents neurodegeneration and suppresses microglial activation in a Parkinson's disease mouse model. Brain penetrant. |

PTIQ Dilution Calculator

PTIQ Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2503 mL | 21.2513 mL | 42.5026 mL | 85.0051 mL | 106.2564 mL |
| 5 mM | 0.8501 mL | 4.2503 mL | 8.5005 mL | 17.001 mL | 21.2513 mL |
| 10 mM | 0.425 mL | 2.1251 mL | 4.2503 mL | 8.5005 mL | 10.6256 mL |
| 50 mM | 0.085 mL | 0.425 mL | 0.8501 mL | 1.7001 mL | 2.1251 mL |
| 100 mM | 0.0425 mL | 0.2125 mL | 0.425 mL | 0.8501 mL | 1.0626 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GDC-0980 (RG7422)
Catalog No.:BCC4992
CAS No.:1032754-93-0
- GNE-477
Catalog No.:BCC8049
CAS No.:1032754-81-6
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- A939572
Catalog No.:BCC5305
CAS No.:1032229-33-6
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Pranlukast
Catalog No.:BCC4827
CAS No.:103177-37-3
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
- L-364,373
Catalog No.:BCC7445
CAS No.:103342-82-1
- 1-Methyl-L-4,5-dihydroorotic acid
Catalog No.:BCC8472
CAS No.:103365-69-1
- GNE-493
Catalog No.:BCC8048
CAS No.:1033735-94-2
- Itol A
Catalog No.:BCN5847
CAS No.:1033747-78-2
- GNF-5837
Catalog No.:BCC3668
CAS No.:1033769-28-6
- Salidroside
Catalog No.:BCN5966
CAS No.:10338-51-9
- Telotristat
Catalog No.:BCC5128
CAS No.:1033805-28-5
A novel compound PTIQ protects the nigral dopaminergic neurones in an animal model of Parkinson's disease induced by MPTP.[Pubmed:21951056]
Br J Pharmacol. 2012 Apr;165(7):2213-27.
BACKGROUND AND PURPOSE: In Parkinson's disease, the dopaminergic neurones in the substantia nigra undergo degeneration. While the exact mechanism for the degeneration is not completely understood, neuronal apoptosis and neuroinflammation are thought to be key contributors. We have recently established that MMP-3 plays crucial roles in dopaminergic cell death and microglial activation. EXPERIMENTAL APPROACH: We tested the effects of 7-hydroxy-6-methoxy-2-propionyl-1,2,3,4-tetrahydroisoquinoline (PTIQ) on expression of MMP-3 and inflammatory molecules and dopaminergic cell death in vitro and in an animal model of Parkinson's disease, and Parkinson's disease-related motor deficits. The pharmacokinetic profile of PTIQ was also evaluated. KEY RESULTS: PTIQ effectively suppressed the production of MMP-3 induced in response to cellular stress in the dopaminergic CATH.a cell line and prevented the resulting cell death. In BV-2 microglial cells activated with lipopolysaccharide, PTIQ down-regulated expression of MMP-3 along with IL-1beta, TNF-alpha and cyclooxygenase-2 and blocked nuclear translocation of NF-kappaB. In the mouse model of Parkinson's disease ,induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), PTIQ attenuated the associated motor deficits, prevented neurodegeneration and suppressed microglial activation in the substantia nigra. Pharmacokinetic analysis showed it was relatively stable against liver microsomal enzymes, did not inhibit the cytochrome p450 isozymes or the hERG ion channel, exhibited no cytotoxicity on liver cells or lethality when administered at 1000 mg kg(-1) and entered the brain rather rapidly yielding a 28% brain:plasma ratio after i.p. injection. CONCLUSIONS AND IMPLICATIONS: These results suggest PTIQ has potential as a candidate drug for disease-modifying therapy for Parkinson's disease.
Studies on the mechanism of action of 1-(2-methyl-3-hydroxy-5-hydroxy-methyl-4-pyridyl)-6,7-dihydroxy-1,2,3, 4- tetrahydroisoquinoline (PTIQ) on blood pressure in the anesthetized dog.[Pubmed:2781139]
Res Commun Chem Pathol Pharmacol. 1989 Jun;64(3):421-33.
The mechanism of action of PTIQ in lowering the arterial blood pressure in the anesthetized dog was studied using pharmacological and physiological techniques. The hypotensive activity of PTIQ was significantly inhibited by pretreatment with bilateral vagotomy, propranolol, prazosin and mecamylamine. Pretreatment with either yohimbine or reserpine had no significant effect on PTIQ activity. PTIQ produced a significant decrease in the force of contraction of myocardial tissue and a transient increase in the aortic blood flow of the anesthetized dog. These results indicate a multiplicity of action of PTIQ in controlling arterial blood pressure.
Syntheses of tetrahydroisoquinoline derivatives that inhibit NO production in activated BV-2 microglial cells.[Pubmed:17980460]
Eur J Med Chem. 2008 Jun;43(6):1160-70.
Seventeen tetrahydroisoquinoline derivatives were designed, synthesized and evaluated for inhibition of NO production in lipopolysaccharide-stimulated BV-2 microglial cells. Compounds 5a, 9c and 11a potently attenuated NO production by >60%, and 5a and 11a inhibited BH4 production by >48% at 100 microM. In particular, N-ethylcarbonyl-7-hydroxy-6-methoxy-1,2,3,4-tetrahydroisoquinoline (11a) reduced NO production by 64% and tetrahydrobiopterin (BH4) production by 49%. Introducing longer alkyl component at C1 or N2 position led to attenuation of the inhibitory effect. It is possible that 11a inhibits NO production by blocking BH4-dependent dimerization of newly synthesized iNOS monomers.


