VX-661F508del CFTR corrector CAS# 1152311-62-0 |
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- PTC124 (Ataluren)
Catalog No.:BCC3881
CAS No.:775304-57-9
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
- VX-809
Catalog No.:BCC3712
CAS No.:936727-05-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1152311-62-0 | SDF | Download SDF |
| PubChem ID | 46199646 | Appearance | Powder |
| Formula | C26H27F3N2O6 | M.Wt | 520.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tezacaftor | ||
| Solubility | DMSO : ≥ 100 mg/mL (192.12 mM) *"≥" means soluble, but saturation unknown. | ||
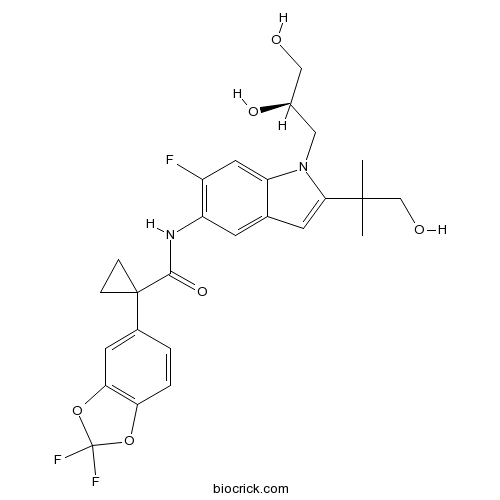
| Chemical Name | 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-N-[1-[(2R)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl]cyclopropane-1-carboxamide | ||
| SMILES | CC(C)(CO)C1=CC2=CC(=C(C=C2N1CC(CO)O)F)NC(=O)C3(CC3)C4=CC5=C(C=C4)OC(O5)(F)F | ||
| Standard InChIKey | MJUVRTYWUMPBTR-MRXNPFEDSA-N | ||
| Standard InChI | InChI=1S/C26H27F3N2O6/c1-24(2,13-33)22-8-14-7-18(17(27)10-19(14)31(22)11-16(34)12-32)30-23(35)25(5-6-25)15-3-4-20-21(9-15)37-26(28,29)36-20/h3-4,7-10,16,32-34H,5-6,11-13H2,1-2H3,(H,30,35)/t16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | VX-661 is a second corrector of F508 del CFTR. | |||||
| Targets | F508 del CFTR | |||||

VX-661 Dilution Calculator

VX-661 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9212 mL | 9.6061 mL | 19.2123 mL | 38.4246 mL | 48.0307 mL |
| 5 mM | 0.3842 mL | 1.9212 mL | 3.8425 mL | 7.6849 mL | 9.6061 mL |
| 10 mM | 0.1921 mL | 0.9606 mL | 1.9212 mL | 3.8425 mL | 4.8031 mL |
| 50 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7685 mL | 0.9606 mL |
| 100 mM | 0.0192 mL | 0.0961 mL | 0.1921 mL | 0.3842 mL | 0.4803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
VX-661, one of vertex derivatives, corrects F508del-CFTR trafficking and increases F508del-CFTR protein activity in vitro [1].
VX-661 treated alone or in combination with ivacaftor have shown to enhance F508del-CFTR trafficking to the cell surface. VX-661 has been at phase 2 study [1].
References:
[1] S. Donaldson, J. Pilewski, M. Griese, Q. Dong, P.-S. Lee, for the VX11–661-101 Study Group. WS7.3 VX-661, an investigational CFTR corrector, in combination with ivacaftor, a CFTR potentiator, in patients with CF and homozygous for the F508Del-CFTR mutation: Interim analysis. Journal of Cystic Fibrosis, Volume 12, Supplement 1, June 2013, Page S14
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
Novel picolinamide-based cystic fibrosis transmembrane regulator modulators: evaluation of WO2013038373, WO2013038376, WO2013038381, WO2013038386 and WO2013038390.[Pubmed:24392786]
Expert Opin Ther Pat. 2014 Jul;24(7):829-37.
Cystic fibrosis (CF) is a genetic disease caused by defects in the CF transmembrane regulator (CFTR) gene, which encodes an epithelial chloride channel. The most common mutation, Delta508CFTR, produces a protein that is misfolded and does not reach the cell membrane. The discovery that partial chloride channel function could be restored by exposure to exogenous chemical stimuli led to the search for selective CFTR modulators. Vertex has led the way in developing N-aryl-1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropanecarboxamide derivatives such as lumacaftor and VX-661, which correct trafficking of Delta508CFTR and partially restore chloride channel activity. Novartis had identified similar activity in a series of picolinamide derivatives such as 3-amino-N-[(2S)-3,3,3-trifluoro-2-hydroxy-2-methylpropyl]-5,6-bis(trifluoromethyl )pyridine-2-carboxamide. A series of five filings from Novartis has expanded on the activity of compounds based on this scaffold and provided compounds with nanomolar potency in cellular assays.
Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression.[Pubmed:25101887]
Sci Transl Med. 2014 Jul 23;6(246):246ra97.
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane regulator (CFTR) that result in reduced anion conductance at the apical membrane of secretory epithelia. Treatment of CF patients carrying the G551D gating mutation with the potentiator VX-770 (ivacaftor) largely restores channel activity and has shown substantial clinical benefit. However, most CF patients carry the DeltaF508 mutation, which impairs CFTR folding, processing, function, and stability. Studies in homozygous DeltaF508 CF patients indicated little clinical benefit of monotherapy with the investigational corrector VX-809 (lumacaftor) or VX-770, whereas combination clinical trials show limited but significant improvements in lung function. We show that VX-770, as well as most other potentiators, reduces the correction efficacy of VX-809 and another investigational corrector, VX-661. To mimic the administration of VX-770 alone or in combination with VX-809, we examined its long-term effect in immortalized and primary human respiratory epithelia. VX-770 diminished the folding efficiency and the metabolic stability of DeltaF508-CFTR at the endoplasmic reticulum (ER) and post-ER compartments, respectively, causing reduced cell surface DeltaF508-CFTR density and function. VX-770-induced destabilization of DeltaF508-CFTR was influenced by second-site suppressor mutations of the folding defect and was prevented by stabilization of the nucleotide-binding domain 1 (NBD1)-NBD2 interface. The reduced correction efficiency of DeltaF508-CFTR, as well as of two other processing mutations in the presence of VX-770, suggests the need for further optimization of potentiators to maximize the clinical benefit of corrector-potentiator combination therapy in CF.


