DofetilideKV11.1 (hERG) channel blocker; inhibits rapid delayed rectifier K+ current (IKr) CAS# 115256-11-6 |
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115256-11-6 | SDF | Download SDF |
| PubChem ID | 71329 | Appearance | Powder |
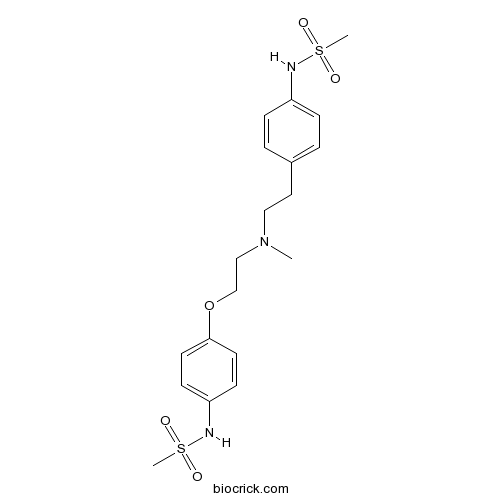
| Formula | C19H27N3O5S2 | M.Wt | 441.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tikosyn, UK 68798 | ||
| Solubility | DMSO : ≥ 100 mg/mL (226.47 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methylamino]ethyl]phenyl]methanesulfonamide | ||
| SMILES | CN(CCC1=CC=C(C=C1)NS(=O)(=O)C)CCOC2=CC=C(C=C2)NS(=O)(=O)C | ||
| Standard InChIKey | IXTMWRCNAAVVAI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective potassium channel blocker. Blocks KV11.1 (hERG) channels; inhibits the rapid delayed-rectifier K+ current (IKr). Displays class III antiarrhythmic properties. |

Dofetilide Dilution Calculator

Dofetilide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2647 mL | 11.3235 mL | 22.647 mL | 45.294 mL | 56.6174 mL |
| 5 mM | 0.4529 mL | 2.2647 mL | 4.5294 mL | 9.0588 mL | 11.3235 mL |
| 10 mM | 0.2265 mL | 1.1323 mL | 2.2647 mL | 4.5294 mL | 5.6617 mL |
| 50 mM | 0.0453 mL | 0.2265 mL | 0.4529 mL | 0.9059 mL | 1.1323 mL |
| 100 mM | 0.0226 mL | 0.1132 mL | 0.2265 mL | 0.4529 mL | 0.5662 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dofetilide is a potassium channel blocker.
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
Antiarrhythmic effect of the Ca(2+)-activated K(+) (SK) channel inhibitor ICA combined with either amiodarone or dofetilide in an isolated heart model of atrial fibrillation.[Pubmed:27722784]
Pflugers Arch. 2016 Nov;468(11-12):1853-1863.
Dose is an important parameter in terms of both efficacy and adverse effects in pharmacological treatment of atrial fibrillation (AF). Both of the class III antiarrhythmics Dofetilide and amiodarone have documented anti-AF effects. While Dofetilide has dose-related ventricular side effects, amiodarone primarily has adverse non-cardiac effects. Pharmacological inhibition of small conductance Ca(2+)-activated K(+) (SK) channels has recently been reported to be antiarrhythmic in a number of animal AF models. In a Langendorff model of acutely induced AF on guinea pig hearts, it was investigated whether a combination of the SK channel blocker N-(pyridin-2-yl)-4-(pyridin-2-yl)thiazol-2-amine (ICA) together with either Dofetilide or amiodarone provided a synergistic effect. The duration of AF was reduced with otherwise subefficacious concentrations of either Dofetilide or amiodarone when combined with ICA, also at a subefficacious concentration. At a concentration level effective as monotherapy, Dofetilide produced a marked increase in the QT interval. This QT prolonging effect was absent when combined with ICA at non-efficacious monotherapy concentrations. The results thereby reveal that combination of subefficacious concentrations of an SK channel blocker and either Dofetilide or amiodarone can maintain anti-AF properties, while the risk of ventricular arrhythmias is reduced.
Practice variation in the re-initiation of dofetilide: An observational study.[Pubmed:28233630]
Int J Cardiol. 2017 Jun 1;236:221-225.
BACKGROUND: Dofetilide is a class III antiarrhythmic drug that has been reported to be safe and efficacious in the treatment of atrial dysrhythmias with a known initial risk of QT prolongation and torsades de pointes (TdP). As a result, the Federal Drug Administration (FDA) mandated in-hospital Dofetilide initiation and adherence to a common dosing protocol. However, there is a lack of clarity on how to manage Dofetilide re-initiation. METHODS: An observational survey was performed including 347 cardiologists in the United States and worldwide to evaluate the deviations from approved manufacturer's protocol during Dofetilide initiation and re-initiation among practicing cardiologists. RESULTS: Most practicing cardiologists were cautious about outpatient Dofetilide use and adhered to the manufacturer's in-patient Dofetilide protocol during de-novo initiation and reported low incidence of TdP in clinical practice. There were substantial differences among practicing cardiologists with deviation from the manufacturer's protocol during re-initiation of Dofetilide. About 21% cardiologists always admitted patients to the hospital while 37% admitted patients <10% of the time for Dofetilide re-initiation. Only 4% reported major adverse events with outpatient Dofetilide re-initiation. There was also wide variation regarding monitoring of electrolytes and QT interval as an outpatient with Dofetilide. CONCLUSION: There is significant practice pattern variation in the use of Dofetilide for the management of AF. This degree of variation noted is concerning and is a reflection of the current lack of substantial clinical evidence in the re-initiation Dofetilide protocol to help direct the provider.
Short- and long-term clinical predictors of pharmacological cardioversion of persistent atrial fibrillation by dofetilide: A retrospective cohort study of 160 patients.[Pubmed:28295387]
Clin Cardiol. 2017 Jul;40(7):474-479.
INTRODUCTION: Dofetilide is a class III antiarrhythmic prescribed to cardiovert persistent atrial fibrillation (AF) to sinus rhythm (SR). HYPOTHESIS: To determine the clinical predictors of cardioversion and readmission in persistent AF patients on Dofetilide. METHODS: We analyzed 160 patients with persistent AF who were started on Dofetilide and followed for 1 year. We examined age, sex, race, hypertension, diabetes, smoking, dyslipidemia, CAD, left ventricular ejection fraction (LVEF), creatinine, BMI and concomitant use of calcium channel blockers (CCB), beta-blockers in a multivariable logistic regression model. We also examined the same predictors in Cox regression model for AF-related readmission within 1 year of follow-up. RESULTS: 13.5% individuals did not convert to SR on Dofetilide. 55.6% converted on the first dose and 83.1% converted by the fourth dose. In multivariable logistic models, dyslipidemia (OR: 2.4, CI: 1.12-5.16) and LVEF (OR: 3.83,CI: 1.37-10.8) were associated with failure to convert with the first dose. Female sex and LVEF also were associated with increased risk of failure to convert at all. Concomitant use of CCB associated with decreased risk of failure to convert to SR. In Cox proportional model, female sex, age <63 years and CAD were associated with increased AF readmission within 1 year. CONCLUSIONS: Dyslipidemia and LVEF <40% were associated with failure to cardiovert after first dose, and female sex and LVEF 40% were related to failure to convert at all on Dofetilide in persistent AF patients. After 1-year follow-up, female sex, known CAD, and age <63 years were associated with increased AF readmissions.
Pharmacologic Conversion during Dofetilide Treatment for Persistent Atrial Fibrillation.[Pubmed:28220940]
Pacing Clin Electrophysiol. 2017 Jun;40(6):667-671.
BACKGROUND: Dofetilide is a pure IKr blocker and is one of the few drugs specifically studied and approved in the United States for the management of persistent atrial fibrillation (AF). Dofetilide has been noted to have a high rate of pharmacologic conversion during initial dosing in prior smaller studies. The intent of the study was to examine the safety of an inpatient loading strategy, and the incidence and patterns of pharmacologic conversion by Dofetilide during the treatment of persistent AF in a large consecutive cohort. METHODS AND RESULTS: This is a retrospective analysis of 308 consecutive patients with persistent AF electively admitted for inpatient Dofetilide loading. The initiation dose of Dofetilide was determined by the creatinine clearance. Overall, 88% (n = 271) successfully completed initiation of Dofetilide and were discharged in sinus rhythm. The most common reason for failure to complete initiation of Dofetilide loading was QTc prolongation in 24 patients (7.8%), and torsade de pointes occurred in three patients (1%). Pharmacologic conversion was observed in 56% (n = 151) after a median of two doses. The rate of pharmacologic conversion based on the final dose was 75%, 9%, and 0% for 500 mcg, 250 mcg, and 125 mcg, respectively (P < 0.05). CONCLUSIONS: Dofetilide is a well-tolerated antiarrhythmic drug with a low incidence of proarrhythmia and an especially high rate of pharmacologic conversion in patients with persistent AF.
[3H]dofetilide binding in SHSY5Y and HEK293 cells expressing a HERG-like K+ channel?[Pubmed:11166283]
Eur J Pharmacol. 2001 Feb 2;412(3):203-12.
The pharmacological characteristics of [3H]Dofetilide binding in SHSY5Y, HEK293 and CHO-K1 cells were examined, and in parallel whole cell recordings used to characterise HERG-like K+ currents. Dofetilide affinity was similar in the human cell lines, SHSY5Y (Kd=99.6 nM) and HEK293 (Kd=102.9 nM), but 10 times lower in CHO-K1 cells (Kd=1200 nM). In contrast, clofilium and E4031 had a similar affinity in all three cell lines, whereas WAY 123,398 had no effect. Electrophysiological studies showed that SHSY5Y cells contained a HERG-like K+ current blocked by application of Dofetilide to either side of the membrane. Block was faster when Dofetilide was applied intracellularly. In contrast, HEK293 and CHO-K1 cells contained no such current, despite the presence of a partial cDNA for HERG in the former. That [3H]Dofetilide is specific for I(Kr)/HERG may be questionable, as HEK293 and CHO-K1 cells contain no such functional K+ current.
Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide.[Pubmed:1501123]
J Pharmacol Exp Ther. 1992 Aug;262(2):809-17.
The delayed K+ current (ik) and its change by Dofetilide was studied in single myocytes from the guinea pig and rabbit heart using the two-electrode voltage clamp technique. In rabbit myocytes, ik consisted of only one component (Kr), which developed for moderate depolarizations and with a fast time course. In guinea pig myocytes, activation consisted of a rapid and a slow component, and the latter (Ks) only became manifest for depolarizations positive to 0 mV. Ks was resistant to block by Dofetilide. Kr, however, was very sensitive: Kd 3.9 x 10(-9) M, Hill coefficient 2.0 (n = 5). The effect was voltage-dependent block increasing at depolarized levels. Block development was time dependent and occurred in two phases: a first fast and voltage-dependent phase was followed by a second much slower phase (time constant of 4.4 +/- 0.48 sec (n = 11). Recovery from block was slower as the membrane potential became more negative. This resulted in the absence of a steady-state frequency-dependent effect at negative membrane potentials. It is concluded that Dofetilide is an efficient blocker of the fast component of ik. The block, as well as recovery, are voltage and time dependent. Block is greater at more depolarized levels, recovery is slower at more hyperpolarized levels.
UK-68,798: a novel, potent and highly selective class III antiarrhythmic agent which blocks potassium channels in cardiac cells.[Pubmed:1988662]
J Pharmacol Exp Ther. 1991 Jan;256(1):318-24.
UK-68,798 increased the duration and effective refractory period of cardiac action potentials recorded in vitro from canine ventricular muscle and Purkinje fibers in a concentration dependent manner from 5 nM to 1 microM. The resting membrane potential, amplitude and maximum upstroke velocity of action potentials were unaffected by UK-68,798, indicating the selective class III antiarrhythmic properties of this agent. UK-68,798 (5 nM-1 microM) increased the effective refractory period of isolated guinea pig papillary muscles at stimulation frequencies of 1 Hz and 5 Hz without influencing the conduction velocity, further confirming that UK-68,798 is devoid of class I antiarrhythmic activity including block of the sodium channel. Studies using single voltage clamped guinea pig ventricular myocytes indicated that UK-68,798 at concentrations of 50 nM and 2 microM blocks a time-dependent K+ current, with no appreciable effects on the time-independent K+ current or the inward calcium current. UK-68,798 is therefore a highly selective K+ channel blocking agent with class III antiarrhythmic properties, a profile that holds considerable promise for the therapy of life-threatening cardiac arrhythmias.


