SiguazodanPDE3 inhibitor CAS# 115344-47-3 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115344-47-3 | SDF | Download SDF |
| PubChem ID | 72124 | Appearance | Powder |
| Formula | C14H16N6O | M.Wt | 284.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
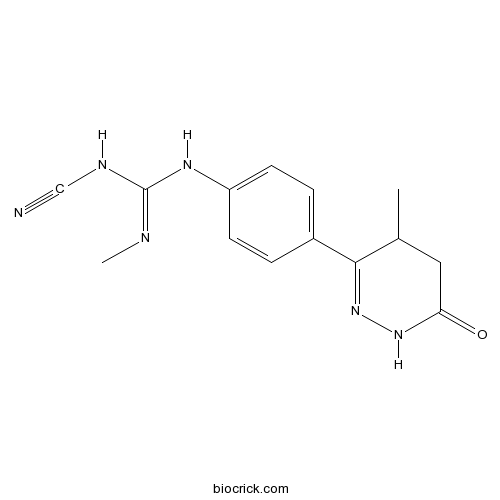
| Chemical Name | 1-cyano-2-methyl-3-[4-(4-methyl-6-oxo-4,5-dihydro-1H-pyridazin-3-yl)phenyl]guanidine | ||
| SMILES | CC1CC(=O)NN=C1C2=CC=C(C=C2)NC(=NC)NC#N | ||
| Standard InChIKey | NUHPODZZKHQQET-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16N6O/c1-9-7-12(21)19-20-13(9)10-3-5-11(6-4-10)18-14(16-2)17-8-15/h3-6,9H,7H2,1-2H3,(H,19,21)(H2,16,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Phosphodiesterase inhibitor, selective for PDE3 (IC50 = 117 nM). |

Siguazodan Dilution Calculator

Siguazodan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5172 mL | 17.5858 mL | 35.1716 mL | 70.3433 mL | 87.9291 mL |
| 5 mM | 0.7034 mL | 3.5172 mL | 7.0343 mL | 14.0687 mL | 17.5858 mL |
| 10 mM | 0.3517 mL | 1.7586 mL | 3.5172 mL | 7.0343 mL | 8.7929 mL |
| 50 mM | 0.0703 mL | 0.3517 mL | 0.7034 mL | 1.4069 mL | 1.7586 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7034 mL | 0.8793 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
Effects of rolipram and siguazodan on the human isolated bronchus and their interaction with isoprenaline and sodium nitroprusside.[Pubmed:8358572]
Br J Pharmacol. 1993 Jul;109(3):774-8.
1 The effects of the selective inhibitors of cyclic AMP phosphodiesterase type IV (rolipram) and type III (Siguazodan) and their interactions with isoprenaline and sodium nitroprusside have been studied in the human isolated bronchus. 2 On bronchi under resting tone rolipram was, in terms of potency (pD2 = 7.77 +/- 0.14, n = 8), very similar to isoprenaline (pD2 = 7.31 +/- 0.12, n = 12) and salbutamol (pD2 = 7.12 +/- 0.17, n = 10) and approximately 10 fold more potent than Siguazodan (pD2 = 6.80 +/- 0.12, n = 6). In terms of efficacy (Emax, expressed as percentage of maximal effect induced by theophylline 3 mM), both rolipram and Siguazodan were less efficient (Emax = 74 +/- 6.7%, n = 8 and 66 +/- 7.5%, n = 6, respectively) than isoprenaline (Emax = 98 +/- 0.4%, n = 12) and salbutamol (Emax = 83 +/- 2.4%, n = 10). 3 During precontraction induced by methacholine (3 x 10(-7) M) or acetylcholine (10(-3) M), concentration-response curves to rolipram and Siguazodan were shifted to the right and maximal effects reduced. Rolipram was more potent than Siguazodan and, in terms of efficacy, it was less active. 4. Rolipram 10(-8) and 10(-7) M but not Siguazodan potentiated the effects of isoprenaline as shown by the shift to the left of the concentration-response curve to isoprenaline. Sodium nitroprusside-induced relaxation was not modified by either drug. 5. These results show that rolipram is a potent relaxant of the human isolated bronchus, potentiating the effects of beta-adrenoceptor stimulation and suggest that, as previously demonstrated in other species(guinea-pig, cow) (Tomkinson et al., 1993), there may be a connection between the beta2-adrenoceptor subtype, which predominate in human airway smooth muscle, and the cyclic AMP phosphodiesterase type IV.
Comparison of responses to siguazodan, rolipram, and zaprinast in the feline pulmonary vascular bed.[Pubmed:11020486]
Eur J Pharmacol. 2000 Oct 13;406(2):233-8.
The present study was undertaken to investigate and compare responses to the cyclic nucleotide phosphodiesterase inhibitors Siguazodan (type III, guanosine 3',5'-cyclic monophosphate (cGMP)-inhibited adenosine 3',5'-cyclic monophosphate (cAMP)), rolipram (type IV, cAMP-specific), and zaprinast (type V, cGMP-specific) in the feline pulmonary vascular bed. When tone in the pulmonary vascular bed was raised to a high steady level with a constant infusion of the thromboxane mimic U46619 (9,11-dideoxy-11, alpha9alpha-epoxymethano prostaglandin F(2alpha)), intralobar injections of the three phosphodiesterase inhibitors caused dose-related decreases in lobar arterial pressure. In terms of relative vasodilator activity, rolipram was more potent at higher doses than Siguazodan, which was more potent than zaprinast. The duration of the pulmonary vasodilator response to zaprinast was shorter than for Siguazodan or rolipram. Furthermore, Siguazodan and rolipram, but not zaprinast, decreased systemic arterial pressure when injected into the perfused lobar artery in the range of doses studied. The present data demonstrate that the three phosphodiesterase inhibitors have potent, long-lasting vasodilator activity in the pulmonary vascular bed of the cat. These data suggest that there is rapid turnover of cAMP and cGMP in the pulmonary circulation and indicate that phosphodiesterase enzyme types III, IV, and V may play an important role in the regulation of vasomotor tone in the feline lung.
The effects of siguazodan, a selective phosphodiesterase inhibitor, on human platelet function.[Pubmed:2158847]
Br J Pharmacol. 1990 Mar;99(3):612-6.
1. The effects of Siguazodan (SK&F 94836) a selective phosphodiesterase (PDE) inhibitor with inotropic and vasodilator activity, were studied on human platelets. 2. Siguazodan selectively inhibited the major cyclic AMP-hydrolysing PDE in human platelet supernatants. The inhibited enzyme has been variously termed cyclic GMP-inhibited PDE or PDE-III. 3. In platelet-rich plasma (PRP), Siguazodan inhibited U46619-induced aggregation more potently than that induced by ADP and collagen. Treatment of the PRP with aspirin had no effect on the potency of Siguazodan. 4. In washed platelets, Siguazodan increased cyclic AMP levels and reduced cytoplasmic free calcium [( Ca2+]i). ADP decreased the ability of Siguazodan to raise cyclic AMP and this may explain its lower potency in inhibiting responses to ADP. 5. Siguazodan has anti-platelet actions over the same concentration range that it is an inotrope and vasodilator.
Rolipram, but not siguazodan or zaprinast, inhibits the excitatory noncholinergic neurotransmission in guinea-pig bronchi.[Pubmed:8162984]
Eur Respir J. 1994 Feb;7(2):306-10.
Theophylline has been reported to inhibit excitatory noncholinergic but not cholinergic-neurotransmission in guinea-pig bronchi. As theophylline might exert this effect through an inhibition of phosphodiesterases (PDE), and since many types of PDE have now been described, the aim of this study was to investigate the effects of three specific inhibitors of PDE on the electrical field stimulation (EFS) of the guinea-pig isolated main bronchus in vitro. The drugs used were Siguazodan, rolipram and zaprinast, which specifically inhibit PDE types, III, IV and V, respectively. Guinea-pig bronchi were stimulated transmurally with biphasic pulses (16 Hz, 1 ms, 320 mA for 10 s) in the presence of indomethacin 10(-6) M and propranolol 10(-6) M. Two successive contractile responses were observed: a rapid cholinergic contraction, followed by a long-lasting contraction due to a local release of neuropeptides from C-fibre endings. Rolipram (10(-9) to 10(-6) M) but not Siguazodan or zaprinast, inhibited the peptidergic contraction in a concentration-dependent manner. Conversely, the cholinergic response was unaffected. Contractile responses induced by exogenous acetylcholine (10(-8) to 10(-3) M) or [Nle10]NKA(4-10) (10(-10) to 10(-6) M) were also unaffected by rolipram, Siguazodan and zaprinast (10(-7) M). These results demonstrate that concentrations of rolipram, similar to those which inhibit PDE, reduce the release of sensory neuropeptides from C-fibre endings, and suggest that the cyclic adenosine monophosphate (AMP) PDE type IV is specifically involved in this effect, as in other anti-inflammatory effects.
Phosphodiesterase inhibitors suppress alpha2-adrenoceptor-mediated 5-hydroxytryptamine release from tracheae of newborn rabbits.[Pubmed:9726632]
Eur J Pharmacol. 1998 Jul 31;354(1):67-71.
The outflow of 5-hydroxytryptamine (5-HT) from isolated tracheae of newborn rabbits was determined by high pressure liquid chromatography with electrochemical detection. This 5-HT outflow reflects release from neuroendocrine epithelial cells of the airway mucosa, as previously shown. Phenylephrine, via alpha2B-adrenoceptors, caused a transient increase in 5-HT outflow, maximally by about 250%, an effect mediated by liberation of intracellular Ca2+, as previously shown. The non-selective phosphodiesterase inhibitor 2-isobutyl-1-methylxanthine (IBMX) concentration-dependently inhibited phenylephrine-induced 5-HT release (completely at 100 microM, IC50: 1.3 microM). Likewise, benzafentrine (inhibitor of phosphodiesterase 3 and 4) and Siguazodan (inhibitor of phosphodiesterase 3) also almost completely inhibited phenylephrine-induced 5-HT release with IC50 values of 1.7 and 4.2 microM, respectively. Rolipram (inhibitor of phosphodiesterase 4), in a concentration of 10 microM, which exceeds more than 10-fold the reported IC50 for phosphodiesterase 4, did not significantly affect phenylephrine-induced 5-HT release. 5-HT release induced by depolarizing concentrations of K+ (45 mM), which largely depends on extracellular Ca2+, was not affected by IBMX. In conclusion, phosphodiesterases, with characteristics of phosphodiesterase 3, appear to play an important role in the control of cyclic nucleotide mediated inhibition of 5-HT release from neuroendocrine epithelial cells.
Photoaffinity labelling of cyclic GMP-inhibited phosphodiesterase (PDE III) in human and rat platelets and rat tissues: effects of phosphodiesterase inhibitors.[Pubmed:7925608]
Eur J Pharmacol. 1994 Jun 15;268(1):105-14.
Ultraviolet irradiation of human platelet cytosol in the presence of 32P-labelled cyclic GMP (cGMP) can specifically label 110, 80, 55, 49 and 38 kDa proteins; the 110 kDa species is the subunit of cGMP-inhibited phosphodiesterase (PDE III) and the 80 kDa species that of cGMP-dependent protein kinase (Tang et al., 1993, Biochem. J. 294, 329). We have now shown that although photolabelling of platelet PDE III was inhibited by unlabelled cGMP, 8-bromo-cGMP and cyclic AMP (cAMP), it was not affected by phosphorothioate analogues of these cyclic nucleotides. Specific concentration-dependent inhibitions of the photolabelling of PDE III were observed with the following PDE inhibitors: trequinsin (IC50 = 13 +/- 2 nM), lixazinone (IC50 = 22 +/- 4 nM), milrinone (IC50 = 56 +/- 12 nM), cilostamide (IC50 = 70 +/- 9 nM), Siguazodan (IC50 = 117 +/- 29 nM) and 3-isobutyl 1-methylxanthine (IBMX) (IC50 = 3950 +/- 22 nM). Thus, measurements of the inhibitory effects of compounds on the photolabelling of platelet PDE III provide a simple quantitative means of investigating their actions at a molecular level that avoids the need to purify the enzyme. Photolabelling of rat platelet lysate or rat heart homogenate by [32P]cGMP showed that the 110 kDa PDE III present in human material was replaced by a 115 kDa protein, labelling of which was also blocked by PDE III inhibitors. Heart and other rat tissues contained much less of this putative 115 kDa PDE III than rat platelets. In contrast, the 80 kDa protein was labelled much less in platelets than in many other rat tissue homogenates (e.g., heart, aorta, uterus and lung). Thus, comparison of the relative amounts of specific photolabelled proteins in different cells may provide an indication of different patterns of cyclic nucleotide action. We compared the abilities of phosphodiesterase inhibitors to block the photolabelling of PDE III in human platelet cytosol and to increase the iloprost-stimulated accumulation of cAMP in intact platelets. Whereas trequinsin (EC50 = 19 +/- 3 nM), lixazinone (EC50 = 122 +/- 8 nM), milrinone (EC50 = 5320 +/- 970 nM) and Siguazodan (EC50 = 18880 +/- 3110 nM) all increased platelet cAMP to the same maximum extent, cilostamide and IBMX increased cAMP further, indicating that they inhibited a PDE isozyme in addition to PDE III.


