NiloticinCAS# 115404-57-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115404-57-4 | SDF | Download SDF |
| PubChem ID | 189300 | Appearance | Cryst. |
| Formula | C30H48O3 | M.Wt | 456.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
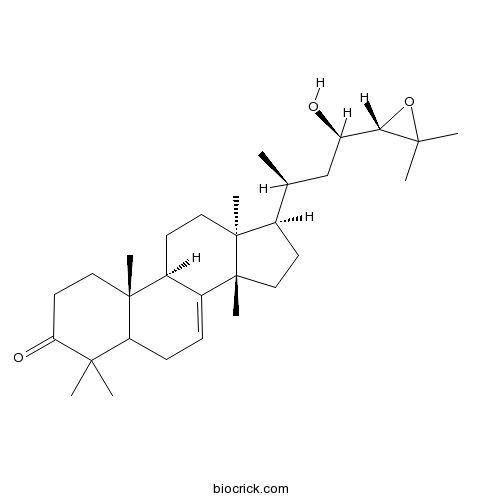
| Chemical Name | (9R,10R,13S,14S,17R)-17-[(2S,4R)-4-[(2S)-3,3-dimethyloxiran-2-yl]-4-hydroxybutan-2-yl]-4,4,10,13,14-pentamethyl-1,2,5,6,9,11,12,15,16,17-decahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC(CC(C1C(O1)(C)C)O)C2CCC3(C2(CCC4C3=CCC5C4(CCC(=O)C5(C)C)C)C)C | ||
| Standard InChIKey | GKQMMZUXYRXFOH-IWPAKXEHSA-N | ||
| Standard InChI | InChI=1S/C30H48O3/c1-18(17-22(31)25-27(4,5)33-25)19-11-15-30(8)21-9-10-23-26(2,3)24(32)13-14-28(23,6)20(21)12-16-29(19,30)7/h9,18-20,22-23,25,31H,10-17H2,1-8H3/t18-,19+,20-,22+,23?,25-,28+,29-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Niloticin can be used as a potential natural mosquitocide, it shows strong larvicidal and pupicidal activities, and shows good mosquitocidal activity against Ae. aegypti. 2. Niloticin can be used against the larvae of A. aegypti as an effective acetylcholinesterase (AChE) inhibitor. |

Niloticin Dilution Calculator

Niloticin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1896 mL | 10.9481 mL | 21.8962 mL | 43.7924 mL | 54.7405 mL |
| 5 mM | 0.4379 mL | 2.1896 mL | 4.3792 mL | 8.7585 mL | 10.9481 mL |
| 10 mM | 0.219 mL | 1.0948 mL | 2.1896 mL | 4.3792 mL | 5.4741 mL |
| 50 mM | 0.0438 mL | 0.219 mL | 0.4379 mL | 0.8758 mL | 1.0948 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.219 mL | 0.4379 mL | 0.5474 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- BI 224436
Catalog No.:BCC5531
CAS No.:1155419-89-8
- Marbofloxacin
Catalog No.:BCC2510
CAS No.:115550-35-1
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- 2'-Hydroxygenistein
Catalog No.:BCN6036
CAS No.:1156-78-1
Ring A-seco limonoids and flavonoids from the Kenyan Vepris uguenensis Engl. and their antioxidant activity.[Pubmed:22898386]
Phytochemistry. 2012 Nov;83:136-43.
Two A-seco-limonoids, accorded the trivial names, uguenensene and uguenensone and a C-7 prenylated flavonoid, uguenenprenol were isolated from Vepris uguenensis (Rutaceae). In addition, 11 known compounds, Niloticin, chisocheton A, kihadalactone A, limonyl acetate, methyl uguenenoate, 7-O-methylaromadenrin, flindersiamine, 8alpha,11-elemodiol, tricoccin S(1)(3) acetate, skimmianine, and lupeol were isolated. The structures of the compounds were elucidated and characterized by spectroscopic analyses (NMR, GC-MS and IR). Antioxidant activity of the isolated compounds showed that uguenenprenol and 7-O-methylaromadenrin are good antioxidant agents. Significantly high antioxidant activity was also exhibited by 8alpha,11-elemodiol, which was 72% at 250 mug mL(-)(1) and 57% at 15.62 mug mL(-)(1) when tested with the deoxyribose method. The two liminoids fit nicely into the biosynthetic pathway from Niloticin to methyl uguenenoate.
Effect of niloticin, a protolimonoid isolated from Limonia acidissima L. (Rutaceae) on the immature stages of dengue vector Aedes aegypti L. (Diptera: Culicidae).[Pubmed:25019220]
Acta Trop. 2014 Nov;139:67-76.
The aim of the present study was to evaluate the mosquitocidal activity of fractions and a compound Niloticin from the hexane extract of Limonia acidissima L. leaves on eggs, larvae and pupae of Aedes aegypti L. (Diptera: Culicidae). In these bioassays, the eggs, larvae and pupae were exposed to concentrations of 2.5, 5.0, 7.5 and 10.0ppm for fractions and 0.5, 1.0, 1.5 and 2.0ppm for compound. After 24h, the mortality was assessed and the LC50 and LC90 values were calculated for larvae and pupae. Per cent ovicidal activity was calculated for eggs after 120h post treatment. Among the sixteen fractions screened, fraction 8 from the hexane extract of L. acidissima generated good mosquitocidal activity against Ae. aegypti. The LC50 and LC90 values of fraction 8 were 4.11, 8.04ppm against Ae. aegypti larvae and 4.19, 8.10ppm against Ae. aegypti pupae, respectively. Further, the isolated compound, Niloticin recorded strong larvicidal and pupicidal activities. The 2ppm concentration of Niloticin showed 100% larvicidal and pupicidal activities in 24h. The LC50 and LC90 values of Niloticin on Ae. aegypti larvae were 0.44, 1.17ppm and on pupae were 0.62, 1.45ppm, respectively. Niloticin presented 83.2% ovicidal activity at 2ppm concentration after 120h post treatment and Niloticin exhibited significant growth disruption and morphological deformities at sub lethal concentrations against Ae. aegypti. The structure of the isolated compound was identified on the basis of single XRD and spectral data ((1)H NMR and (13)C NMR) and compared with literature spectral data. The results indicate that Niloticin could be used as a potential natural mosquitocide.


