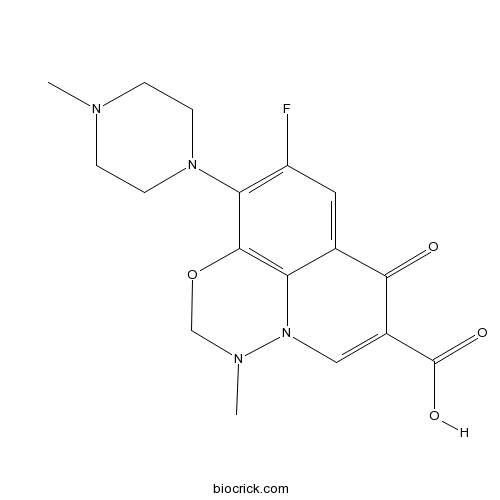
MarbofloxacinFluoroquinolone antibiotic for veterinary use CAS# 115550-35-1 |
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115550-35-1 | SDF | Download SDF |
| PubChem ID | 60651 | Appearance | Powder |
| Formula | C17H19FN4O4 | M.Wt | 362.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 3.8 mg/mL (10.49 mM) *"≥" means soluble, but saturation unknown. | ||
| SMILES | CN1CCN(CC1)C2=C(C=C3C4=C2OCN(N4C=C(C3=O)C(=O)O)C)F | ||
| Standard InChIKey | BPFYOAJNDMUVBL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H19FN4O4/c1-19-3-5-21(6-4-19)14-12(18)7-10-13-16(14)26-9-20(2)22(13)8-11(15(10)23)17(24)25/h7-8H,3-6,9H2,1-2H3,(H,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Marbofloxacin is a potent antibiotic of which depends upon its inhibition of DNA-gyrase. Marbofloxacin is a synthetic, broad spectrum bactericidal agent.
Target: DNA-gyrase
Marbofloxacin is a third-generation fluoroquinolone for veterinary use, the antimicrobial of which depends upon its inhibition of DNA-gyrase and topoisomerase IV. With a broad spectrum bactericidal activity and good efficacy, marbofloxacin is indicated for dermatological, respiratory and urinary tract infections due to both Gram-positive and Gram-negative bacteria and Mycoplasma [1].
Administration of Marbofloxacin at 6 mg/kg once daily for 7 days in a Staphylococcus aureus infection in tissue cages in ponies is not effective for the elimination of S. aureus infections from secluded sites [2]. The pharmacokinetic properties of marbofloxacin were investigated in 6 horses after i.v., subcutaneous and oral administration of a single dose of 2 mg/kg bwt and the minimal inhibitory concentrations (MIC) assessed for bacteria isolated from equine infectious pathologies. The clearance of marbofloxacin was mean +/- s.d. 0.25 +/- 0.05 l/kg/h and the terminal half-life 756 +/- 1.99 h. The marbofloxacin absolute bioavailabilities after subcutaneous and oral administration were 98 +/- 11% and 62 +/- 8%, respectively. Considering the breakpoint values of efficacy indices for fluoroquinolones, a marbofloxacin dosage regimen of 2 mg/kg bwt/24 h by i.v., subcutaneous or oral routes was more appropriate for enterobacteriaceae than for S. aureus [3].
Toxicity: cramps; vomiting; anorexia; soft stools; diarrhoea References: | |||||

Marbofloxacin Dilution Calculator

Marbofloxacin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7597 mL | 13.7984 mL | 27.5969 mL | 55.1937 mL | 68.9922 mL |
| 5 mM | 0.5519 mL | 2.7597 mL | 5.5194 mL | 11.0387 mL | 13.7984 mL |
| 10 mM | 0.276 mL | 1.3798 mL | 2.7597 mL | 5.5194 mL | 6.8992 mL |
| 50 mM | 0.0552 mL | 0.276 mL | 0.5519 mL | 1.1039 mL | 1.3798 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.276 mL | 0.5519 mL | 0.6899 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Marbofloxacin is a fluorinated, quinolone antibacterial.
- BI 224436
Catalog No.:BCC5531
CAS No.:1155419-89-8
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- 2'-Hydroxygenistein
Catalog No.:BCN6036
CAS No.:1156-78-1
- 4-Androstenediol
Catalog No.:BCC8692
CAS No.:1156-92-9
- Cinnamyl coumarate
Catalog No.:BCN7739
CAS No.:115610-30-5
- Cinnamyl isoferulate
Catalog No.:BCN7718
CAS No.:115610-31-6
- Cinnamyl caffeate
Catalog No.:BCN7721
CAS No.:115610-32-7
- H-DL-Pro-NH2
Catalog No.:BCC3027
CAS No.:115630-49-4
- B2
Catalog No.:BCC7505
CAS No.:115687-05-3
- Daidzein dimethyl ether
Catalog No.:BCN6761
CAS No.:1157-39-7
- Adonifoline
Catalog No.:BCN2056
CAS No.:115712-88-4
- 6'-O-beta-D-Glucosylgentiopicroside
Catalog No.:BCN2814
CAS No.:115713-06-9
- 1-O-galloyl-6-O-cinnamoylglucose
Catalog No.:BCN8264
CAS No.:115746-69-5
Impact of Low and High Doses of Marbofloxacin on the Selection of Resistant Enterobacteriaceae in the Commensal Gut Flora of Young Cattle: Discussion of Data from 2 Study Populations.[Pubmed:28072925]
Foodborne Pathog Dis. 2017 Mar;14(3):152-159.
In the context of requested decrease of antimicrobial use in veterinary medicine, our objective was to assess the impact of two doses of Marbofloxacin administered on young bulls (YBs) and veal calves (VCs) treated for bovine respiratory disease, on the total population of Enterobacteriaceae in gut flora and on the emergence of resistant Enterobacteriaceae. In two independent experiments, 48 YBs from 6 commercial farms and 33 VCs previously colostrum deprived and exposed to cefquinome were randomly assigned to one of the three groups LOW, HIGH, and Control. In LOW and HIGH groups, animals received a single injection of, respectively, 2 and 10 mg/kg Marbofloxacin. Feces were sampled before treatment, and at several times after treatment. Total and resistant Enterobacteriaceae enumerating were performed by plating dilutions of fecal samples on MacConkey agar plates that were supplemented or not with quinolone. In YBs, Marbofloxacin treatment was associated with a transient decrease in total Enterobacteriaceae count between day (D)1 and D3 after treatment. Total Enterobacteriaceae count returned to baseline between D5 and D7 in all groups. None of the 48 YBs harbored Marbofloxacin-resistant Enterobacteriaceae before treatment. After treatment, 1 out of 20 YBs from the Control group and 1 out of 14 YBs from the HIGH group exhibited Marbofloxacin-resistant Enterobacteriaceae. In VCs, the rate of fluoroquinolone-resistant Enterobacteriaceae significantly increased after low and high doses of Marbofloxacin treatment. However, the effect was similar for the two doses, which was probably related to the high level of resistant Enterobacteriaceae exhibited before treatment. Our results suggest that a single treatment with 2 or 10 mg/kg Marbofloxacin exerts a moderate selective pressure on commensal Enterobacteriaceae in YBs and in VCs. A fivefold decrease of Marbofloxacin regimen did not affect the selection of resistances among commensal bacteria.
Survey of susceptibility to marbofloxacin in bacteria isolated from diseased pigs in Europe.[Pubmed:28348142]
Vet Rec. 2017 Jun 17;180(24):591.
A monitoring programme of Marbofloxacin susceptibility of bacteria from Europe causing respiratory tract infection and meningitis in pigs has been active since 1994 and 2002, respectively. Monitoring digestive, metritis and urinary tract infection (UTI) in pigs has been active since 2005 and susceptibility results until 2013 are presented. Minimum inhibitory concentration (MIC) was determined by broth microdilution. For MIC interpretation, Vetoquinol-evaluated breakpoints were applied. For digestive pathogens, Escherichia coli and Salmonella species (1717 and 300 isolates, respectively) exhibited 7.5 per cent resistance in E coli and no resistance in Salmonella species. Similarly, E coli from metritis (369 isolates) had 7.0 per cent resistance to Marbofloxacin. However, E coli from UTI (633 isolates) had higher resistance (10.4 per cent). For Streptococcus suis causing meningitis (585 isolates), Marbofloxacin susceptibility was very high with only 0.5 per cent resistance and 0.4 per cent resistance was observed with S suis causing respiratory disease (729 isolates). Other respiratory pathogens were also highly susceptible to Marbofloxacin with no resistance in Actinobacillus pleuropneumoniae (647 isolates) or Bordetella bronchiseptica (504 isolates), 0.1 per cent resistance in Pasteurella multocida (1373 isolates) and 1.4 per cent resistance in Haemophilus parasuis (145 isolates). There was no apparent change in Marbofloxacin MIC over time for any bacterial pathogen based on MIC50/90 These data confirm previously published MIC results from porcine and other animal infections.
Factors influencing the potency of marbofloxacin for pig pneumonia pathogens Actinobacillus pleuropneumoniae and Pasteurella multocida.[Pubmed:28113129]
Res Vet Sci. 2017 Apr;111:93-98.
For the pig respiratory tract pathogens, Actinobacillus pleuropneumoniae and Pasteurella multocida, Minimum Inhibitory Concentration (MIC) of Marbofloxacin was determined in recommended broths and pig serum at three inoculum strengths. MICs in both growth matrices increased progressively from low, through medium to high starting inoculum counts, 10(4), 10(6) and 10(8)CFU/mL, respectively. P. multocida MIC ratios for high:low inocula were 14:4:1 for broth and 28.2:1 for serum. Corresponding MIC ratios for A. pleuropneumoniae were lower, 4.1:1 (broth) and 9.2:1 (serum). MIC high:low ratios were therefore both growth matrix and bacterial species dependent. The effect of alterations to the chemical composition of broths and serum on MIC were also investigated. Neither adjusting broth or serum pH in six increments over the range 7.0 to 8.0 nor increasing calcium and magnesium concentrations of broth in seven incremental steps significantly affected MICs for either organism. In time-kill studies, the killing action of Marbofloxacin had the characteristics of concentration dependency against both organisms in both growth matrices. It is concluded that MIC and time-kill data for Marbofloxacin, generated in serum, might be preferable to broth data, for predicting dosages of Marbofloxacin for clinical use.
Potency of marbofloxacin for pig pneumonia pathogens Actinobacillus pleuropneumoniae and Pasteurella multocida: Comparison of growth media.[Pubmed:27940285]
Res Vet Sci. 2017 Apr;111:43-48.
Pharmacodynamic properties of Marbofloxacin were established for six isolates each of the pig respiratory tract pathogens, Actinobacillus pleuropneumoniae and Pasteurella multocida. Three in vitro indices of potency were determined; Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and Mutant Prevention Concentration (MPC). For MIC determination Clinical Laboratory Standards Institute guidelines were modified in three respects: (1) comparison was made between two growth media, an artificial broth and pig serum; (2) a high inoculum count was used to simulate heavy clinical bacteriological loads; and (3) five overlapping sets of two-fold dilutions were used to improve accuracy of determinations. Similar methods were used for MBC and MPC estimations. MIC and MPC serum:broth ratios for A. pleuropneumoniae were 0.79:1 and 0.99:1, respectively, and corresponding values for P. multocida were 1.12:1 and 1.32:1. Serum protein binding of Marbofloxacin was 49%, so that fraction unbound (fu) serum MIC values were significantly lower than those predicted by correction for protein binding; fu serum:broth MIC ratios were 0.40:1 (A. pleuropneumoniae) and 0.50:1 (P. multocida). For broth, MPC:MIC ratios were 13.7:1 (A. pleuropneumoniae) and 14.2:1 (P. multocida). Corresponding ratios for serum were similar, 17.2:1 and 18.8:1, respectively. It is suggested that, for dose prediction purposes, serum data might be preferable to potency indices measured in broths.


