BI 224436CAS# 1155419-89-8 |
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1155419-89-8 | SDF | Download SDF |
| PubChem ID | 66561902 | Appearance | Powder |
| Formula | C27H26N2O4 | M.Wt | 442.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (112.99 mM) *"≥" means soluble, but saturation unknown. | ||
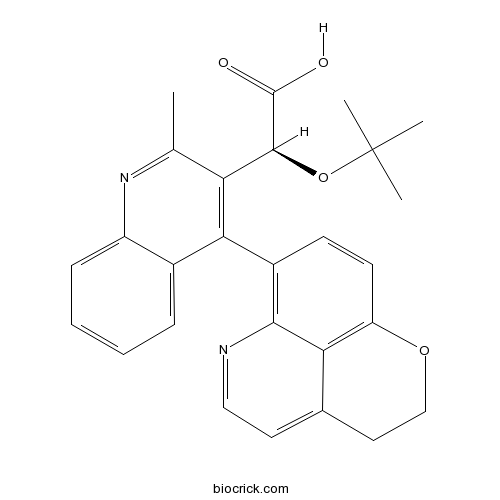
| SMILES | CC1=NC2=CC=CC=C2C(=C1C(C(=O)O)OC(C)(C)C)C3=C4C5=C(C=C3)OCCC5=CC=N4 | ||
| Standard InChIKey | MIXIIJCBELCMCZ-VWLOTQADSA-N | ||
| Standard InChI | InChI=1S/C27H26N2O4/c1-15-21(25(26(30)31)33-27(2,3)4)23(17-7-5-6-8-19(17)29-15)18-9-10-20-22-16(12-14-32-20)11-13-28-24(18)22/h5-11,13,25H,12,14H2,1-4H3,(H,30,31)/t25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

BI 224436 Dilution Calculator

BI 224436 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2598 mL | 11.2992 mL | 22.5984 mL | 45.1967 mL | 56.4959 mL |
| 5 mM | 0.452 mL | 2.2598 mL | 4.5197 mL | 9.0393 mL | 11.2992 mL |
| 10 mM | 0.226 mL | 1.1299 mL | 2.2598 mL | 4.5197 mL | 5.6496 mL |
| 50 mM | 0.0452 mL | 0.226 mL | 0.452 mL | 0.9039 mL | 1.1299 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.226 mL | 0.452 mL | 0.565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BI 224436 is a novel HIV-1 noncatalytic site integrase inhibitor with EC50 values of less than 15 nM against different HIV-1 laboratory strains.
In Vitro:BI 224436 has cellular cytotoxicity of more than 90 μM. BI 224436 has a low, 2.1-fold decrease in antiviral potency in the presence of 50% human serum. BI 224436 retains full antiviral activity against recombinant viruses encoding INSTI resistance substitutions N155S, Q148H, and E92Q. BI 224436 displays an additive effect in combination with most approved antiretrovirals, including INSTIs. BI 224436 has drug-like in vitro absorption, distribution, metabolism, and excretion (ADME) properties, including Caco-2 cell permeability, solubility, and low cytochrome P450 inhibition[1].
In Vivo:BI 224436 exhibits excellent pharmacokinetic profiles in rat (clearance as a percentage of hepatic flow [CL], 0.7%; bioavailability [F], 54%), monkey (CL, 23%; F, 82%), and dog (CL, 8%; F, 81%)[1].
References:
[1]. Fenwick C, et al. Preclinical profile of BI 224436, a novel HIV-1 non-catalytic-site integrase inhibitor. Antimicrob Agents Chemother. 2014 Jun;58(6):3233-44.
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- Marbofloxacin
Catalog No.:BCC2510
CAS No.:115550-35-1
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- 2'-Hydroxygenistein
Catalog No.:BCN6036
CAS No.:1156-78-1
- 4-Androstenediol
Catalog No.:BCC8692
CAS No.:1156-92-9
- Cinnamyl coumarate
Catalog No.:BCN7739
CAS No.:115610-30-5
- Cinnamyl isoferulate
Catalog No.:BCN7718
CAS No.:115610-31-6
- Cinnamyl caffeate
Catalog No.:BCN7721
CAS No.:115610-32-7
- H-DL-Pro-NH2
Catalog No.:BCC3027
CAS No.:115630-49-4
- B2
Catalog No.:BCC7505
CAS No.:115687-05-3
- Daidzein dimethyl ether
Catalog No.:BCN6761
CAS No.:1157-39-7
- Adonifoline
Catalog No.:BCN2056
CAS No.:115712-88-4
- 6'-O-beta-D-Glucosylgentiopicroside
Catalog No.:BCN2814
CAS No.:115713-06-9
Preclinical profile of BI 224436, a novel HIV-1 non-catalytic-site integrase inhibitor.[Pubmed:24663024]
Antimicrob Agents Chemother. 2014 Jun;58(6):3233-44.
BI 224436 is an HIV-1 integrase inhibitor with effective antiviral activity that acts through a mechanism that is distinct from that of integrase strand transfer inhibitors (INSTIs). This 3-quinolineacetic acid derivative series was identified using an enzymatic integrase long terminal repeat (LTR) DNA 3'-processing assay. A combination of medicinal chemistry, parallel synthesis, and structure-guided drug design led to the identification of BI 224436 as a candidate for preclinical profiling. It has antiviral 50% effective concentrations (EC50s) of <15 nM against different HIV-1 laboratory strains and cellular cytotoxicity of >90 muM. BI 224436 also has a low, approximately 2.1-fold decrease in antiviral potency in the presence of 50% human serum and, by virtue of a steep dose-response curve slope, exhibits serum-shifted EC95 values ranging between 22 and 75 nM. Passage of virus in the presence of inhibitor selected for either A128T, A128N, or L102F primary resistance substitutions, all mapping to a conserved allosteric pocket on the catalytic core of integrase. BI 224436 also retains full antiviral activity against recombinant viruses encoding INSTI resistance substitutions N155S, Q148H, and E92Q. In drug combination studies performed in cellular antiviral assays, BI 224436 displays an additive effect in combination with most approved antiretrovirals, including INSTIs. BI 224436 has drug-like in vitro absorption, distribution, metabolism, and excretion (ADME) properties, including Caco-2 cell permeability, solubility, and low cytochrome P450 inhibition. It exhibited excellent pharmacokinetic profiles in rat (clearance as a percentage of hepatic flow [CL], 0.7%; bioavailability [F], 54%), monkey (CL, 23%; F, 82%), and dog (CL, 8%; F, 81%). Based on the excellent biological and pharmacokinetic profile, BI 224436 was advanced into phase 1 clinical trials.
Discovery of BI 224436, a Noncatalytic Site Integrase Inhibitor (NCINI) of HIV-1.[Pubmed:24900852]
ACS Med Chem Lett. 2014 Jan 22;5(4):422-7.
An assay recapitulating the 3' processing activity of HIV-1 integrase (IN) was used to screen the Boehringer Ingelheim compound collection. Hit-to-lead and lead optimization beginning with compound 1 established the importance of the C3 and C4 substituent to antiviral potency against viruses with different aa124/aa125 variants of IN. The importance of the C7 position on the serum shifted potency was established. Introduction of a quinoline substituent at the C4 position provided a balance of potency and metabolic stability. Combination of these findings ultimately led to the discovery of compound 26 (BI 224436), the first NCINI to advance into a phase Ia clinical trial.


