Carmoxirole hydrochlorideSelective, peripherally acting D2-like agonist CAS# 115092-85-8 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115092-85-8 | SDF | Download SDF |
| PubChem ID | 9822866 | Appearance | Powder |
| Formula | C24H27ClN2O2 | M.Wt | 410.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | EMD 45609 | ||
| Solubility | Soluble to 100 mM in DMSO | ||
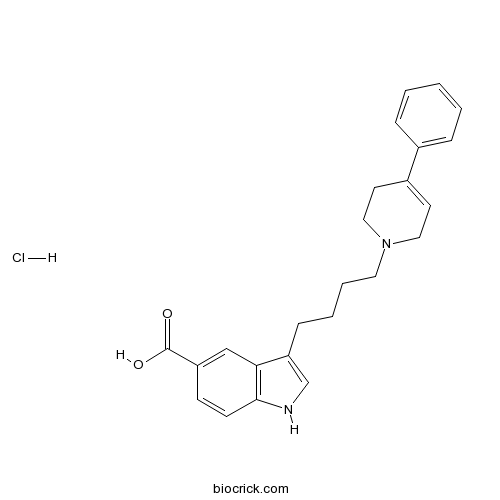
| Chemical Name | 3-[4-(4-phenyl-3,6-dihydro-2H-pyridin-1-yl)butyl]-1H-indole-5-carboxylic acid;hydrochloride | ||
| SMILES | C1CN(CC=C1C2=CC=CC=C2)CCCCC3=CNC4=C3C=C(C=C4)C(=O)O.Cl | ||
| Standard InChIKey | LRJUHOBITQUXIO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H26N2O2.ClH/c27-24(28)20-9-10-23-22(16-20)21(17-25-23)8-4-5-13-26-14-11-19(12-15-26)18-6-2-1-3-7-18;/h1-3,6-7,9-11,16-17,25H,4-5,8,12-15H2,(H,27,28);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, peripherally acting dopamine D2 receptor agonist. Modulates noradrenalin release and sympathetic activation. Displays antihypertensive properties in vivo. |

Carmoxirole hydrochloride Dilution Calculator

Carmoxirole hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4334 mL | 12.1672 mL | 24.3345 mL | 48.6689 mL | 60.8361 mL |
| 5 mM | 0.4867 mL | 2.4334 mL | 4.8669 mL | 9.7338 mL | 12.1672 mL |
| 10 mM | 0.2433 mL | 1.2167 mL | 2.4334 mL | 4.8669 mL | 6.0836 mL |
| 50 mM | 0.0487 mL | 0.2433 mL | 0.4867 mL | 0.9734 mL | 1.2167 mL |
| 100 mM | 0.0243 mL | 0.1217 mL | 0.2433 mL | 0.4867 mL | 0.6084 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3994
CAS No.:115006-86-5
- 9-Phenylcarbazole
Catalog No.:BCN2259
CAS No.:1150-62-5
- Linalyl Acetate
Catalog No.:BCC8200
CAS No.:115-95-7
- Ambenonium dichloride
Catalog No.:BCC6630
CAS No.:115-79-7
- Sinomenine
Catalog No.:BCN6265
CAS No.:115-53-7
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
Dopamine receptor modulation of noradrenaline release by carmoxirole in human cortical kidney slices.[Pubmed:8097997]
Eur J Clin Pharmacol. 1993;44 Suppl 1:S47-9.
The effect of the dopamine D2-receptor agonist carmoxirole on noradrenaline release was investigated in human and rat cortical kidney slices. After preincubation with 3H-noradrenaline, the slices were electrically stimulated at 5 Hz in superfusion chambers, and the stimulation-induced (S-I) outflow of radioactivity was taken as the index of noradrenaline release. In human but not in rat cortical kidney slices, carmoxirole (0.03 microM) inhibited the S-I outflow of radioactivity. Carmoxirole (0.3 microM) also failed to inhibit the S-I outflow of radioactivity from human kidney slices. When alpha-adrenoceptors were blocked by the non-selective alpha-adrenoceptor antagonist phentolamine (1 microM), carmoxirole (0.03 microM, 0.3 microM) inhibited S-I outflow to a similar extent. The inhibitory effect of carmoxirole (0.03 microM) was prevented by the D2-receptor antagonist (-)-sulpiride (10 microM) but not by the D1-receptor antagonist SCH 23390 (1 microM) in human kidney slices. Phentolamine (1 microM) by itself induced a five-fold greater enhancement of the S-I outflow of radioactivity in rat than in human cortical kidney slices. The data suggest that activation of prejunctional D2-receptors by carmoxirole inhibits noradrenaline release from human renal sympathetic nerves. Carmoxirole in higher concentrations (0.3 microM) blocks inhibitory prejunctional alpha-autoreceptors, which seems to mask the inhibitory D2-receptor mediated effect. The different effects of phentolamine and carmoxirole in human and rat kidney may indicate a difference of the prejunctional alpha-autoreceptor mechanism in the two species.
Pharmacological basis for antihypertensive therapy with a novel dopamine agonist.[Pubmed:1356783]
Eur Heart J. 1992 Sep;13 Suppl D:129-35.
In the past, nearly all major mechanisms involved in the regulation of blood pressure have become targets of antihypertensive drugs. They include the brain stem with its neuronal circuits of central cardiovascular regulation, the sympathetic neuro-effector system, the kidney, the renin angiotensin aldosterone system and the vascular smooth muscle cell. There are various ways of influencing the function of the sympathetic nervous system, but the clinical potential of one mechanism of action has not yet been explored in detail. Drugs that inhibit noradrenaline release through stimulation of inhibitory receptors located at adrenergic nerve terminals in the cardiovascular system (inhibitory presynaptic receptors) are not available for the treatment of hypertension. Among the multiple presynaptic receptors, dopamine receptors which belong to the dopamine2 subtype, are of particular interest. Carmoxirole is a novel indole derivative with a potent agonist action selective for dopamine2-receptors of the periphery. Experimental evidence shows that carmoxirole lowers blood pressure in various models of hypertension mainly or exclusively through inhibition of noradrenaline release from sympathetic nerve endings. This effect of carmoxirole is mediated by presynaptic dopamine receptors with the characteristic that release inhibition is restricted to low rates of sympathetic nerve discharge.


