CNQXCAS# 115066-14-3 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- FMK
Catalog No.:BCC1580
CAS No.:821794-92-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115066-14-3 | SDF | Download SDF |
| PubChem ID | 3721046 | Appearance | Powder |
| Formula | C9H4N4O4 | M.Wt | 232.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FG9065 | ||
| Solubility | DMSO : ≥ 30 mg/mL (129.23 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
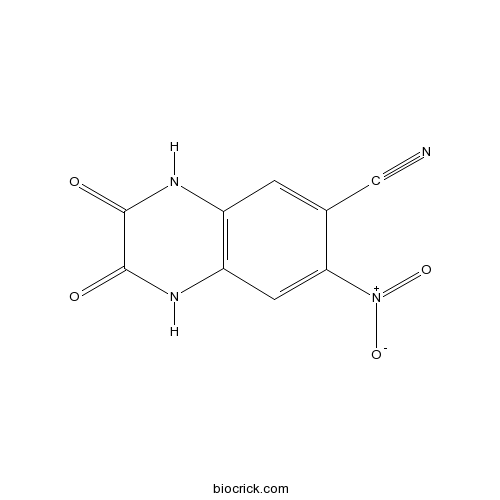
| Chemical Name | 7-nitro-2,3-dioxo-1,4-dihydroquinoxaline-6-carbonitrile | ||
| SMILES | C1=C(C(=CC2=C1NC(=O)C(=O)N2)[N+](=O)[O-])C#N | ||
| Standard InChIKey | RPXVIAFEQBNEAX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H4N4O4/c10-3-4-1-5-6(2-7(4)13(16)17)12-9(15)8(14)11-5/h1-2H,(H,11,14)(H,12,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent AMPA/kainate receptor antagonist. Also antagonist at glycine modulatory site on NMDA receptor complex. CNQX disodium salt also available. |

CNQX Dilution Calculator

CNQX Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3074 mL | 21.5369 mL | 43.0737 mL | 86.1475 mL | 107.6844 mL |
| 5 mM | 0.8615 mL | 4.3074 mL | 8.6147 mL | 17.2295 mL | 21.5369 mL |
| 10 mM | 0.4307 mL | 2.1537 mL | 4.3074 mL | 8.6147 mL | 10.7684 mL |
| 50 mM | 0.0861 mL | 0.4307 mL | 0.8615 mL | 1.7229 mL | 2.1537 mL |
| 100 mM | 0.0431 mL | 0.2154 mL | 0.4307 mL | 0.8615 mL | 1.0768 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CNQX (FG9065) is a potent AMPA/kainate receptor antagonist.
In Vitro:In rat hippocampal slices bathed in Mg2+-free medium, 10 μM CNQX reversibly blocks responses to a-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA), quisqualate and kainate but not NMDA[1]. Superfusion of hippocampal slices with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 2-5 μM) reversibly blocks the Schaffer collateral and mossy fibre excitatory postsynaptic potential (EPSP), while sparing the fast and slow GABA-mediated inhibition[2]. CNQX (1-5 μM) produces a selective and dose-dependent reduction in the amplitude of the monosynaptic component of the DR-VRR recorded from lumbar spinal segments[3]. CNQX-mediated depolarizations are mediated by AMPAR but not kainate receptors in TRN neurons[4].
In Vivo:The bilateral infusion of CNQX (0.5 or 1.25 μg) into the amygdala or dorsal hippocampus 10 min prior to a retention test partially blocks the expression of stepdown inhibitory avoidance in rats 24 h after training. CNQX causes a complete blockade at a dose of 0.5 μg[5].
References:
[1]. Blake JF, et al. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rathippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182-6.
[2]. Neuman RS, et al. Blockade of excitatory synaptic transmission by 6-cyano-7-nitroquinoxaline-2,3-dione(CNQX) in the hippocampus in vitro. Neurosci Lett. 1988 Sep 23;92(1):64-8.
[3]. Alford S, et al. CNQX and DNQX block non-NMDA synaptic transmission but not NMDA-evoked locomotion in lamprey spinal cord. Brain Res. 1990 Jan 8;506(2):297-302.
[4]. Lee SH, et al. Selective excitatory actions of DNQX and CNQX in rat thalamic neurons. J Neurophysiol. 2010 Apr;103(4):1728-34.
[5]. Kim M, et al. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behav Neural Biol. 1993 Jan;59(1):5-8.
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3994
CAS No.:115006-86-5
- 9-Phenylcarbazole
Catalog No.:BCN2259
CAS No.:1150-62-5
- Linalyl Acetate
Catalog No.:BCC8200
CAS No.:115-95-7
- Ambenonium dichloride
Catalog No.:BCC6630
CAS No.:115-79-7
- Sinomenine
Catalog No.:BCN6265
CAS No.:115-53-7
- Azacyclonol
Catalog No.:BCC4761
CAS No.:115-46-8
- Bromophenol Blue
Catalog No.:BCC8029
CAS No.:115-39-9
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
CNQX facilitates inhibitory synaptic transmission in rat hypoglossal nucleus.[Pubmed:26902496]
Brain Res. 2016 Apr 15;1637:71-80.
6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX) is a most commonly used antagonist of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor in the central nervous system. During the past two decades, studies had demonstrated that CNQX could partially activate AMPA receptors that are located on the hippocampal and cerebellar interneurons, thus subsequently leading to the facilitation of inhibitory transmission. However, whether CNQX could enhance inhibitory synaptic transmission in the hypoglossal nucleus remains elusive. Here, using whole-cell patch-clamp recording in the brainstem slice, we showed that CNQX greatly increased both frequency and amplitude of spontaneous inhibitory postsynaptic currents in the hypoglossal motoneurons, whereas D-(-)-2-Amino-5-phosphonopentanoic acid (D-AP5), N-methyl-D-aspartate (NMDA) receptor antagonist, had no effect on inhibitory synaptic transmission. Application of bicuculline and strychnine further identified that CNQX not only increased GABAergic sIPSCs but also glycinergic one in these motoneurons. Similar enhancement of inhibitory transmission was observed with application of 6, 7-dinitroquinoxaline-2, 3-dione (DNQX), a quinoxaline derivative of CNQX, but not with application of GYKI 53655, a non-competitive antagonist of AMPA receptor. In the presence of tetradotoxin, the effect of CNQX on sIPSCs was abolished, suggesting that an increase in presynaptic interneuron spike firing rate induced by CNQX was responsible for the facilitation of sIPSCs. Taken together, these results demonstrated that the excitatory effect of CNQX on presynaptic interneurons triggered enhancement of both GABAergic and glycinergic synaptic transmission within the rat hypoglossal nucleus.
Exposure to sub-chronic unpredictable stress accounts for antidepressant-like effects in hamsters treated with BDNF and CNQX.[Pubmed:26409118]
Brain Res Bull. 2015 Sep;118:65-77.
Recent evidences indicate that cerebral neurotrophic factors like vascular endothelial growth factor plus signaling pathways of the glutamatergic neuroreceptor system (L-Glu) are determinant modulators of depression-like states. In the present study, the type of interaction(s) exerted by the AMPAergic antagonist, 6-cyano-7-nitroquinoxalin-2,3-dione (CNQX) and the brain derived neurotrophic factor (BDNF) on depression-like behaviors in hamsters (Mesocricetus auratus) were investigated. Sub-chronic administration of BDNF in the hippocampal dentate gyrus (DG) of stressed hamsters was responsible for very evident (p<0.001) sucrose consumption along with notably elevated swimming bouts and reduced immobility states in the forced swim test (FST). Meanwhile, CNQX displayed evident anxiolytic actions in the elevated plus maze (EPM) as shown by marked (p<0.01) increases of movements to and from both arms. Interestingly cerebral neurodegeneration events, which are viewed during depression states, were reduced following treatment with both compounds. Contextually, marked mRNA expression levels of the BDNF receptor (tropomyosin-related kinase B; TrkB) were detected in DG and the oriens-pyramidalis of HIP (Or-Py) while a moderate (p<0.05) up-regulation was registered in the amygdalar central nucleus (CeA) and the hypothalamic ventromedial nucleus (VMH) of hamsters treated with BDNF. Similarly, this treatment caused moderate increases of the major stress protein (Hsp70) in DG and Or-Py. Conversely, while CNQX induced similar TrkB expression levels, it instead accounted for a moderate reduction of Hsp70 mRNAs in the same brain areas. Overall these results support crucial roles played by BDNF and AMPAergic neurosignaling mechanisms during distinct adaptive responses of depression- and anxiety-like states in hamsters.
Immediate and delayed treatment with gabapentin, carbamazepine and CNQX have almost similar impact on cognitive functions and behavior in the lithium-pilocarpine model in rats.[Pubmed:27426469]
Pharmacol Biochem Behav. 2016 Sep;148:128-35.
In the present study, we aimed to investigate the effects of immediate and delayed treatment with intracerebroventricular (i.c.v.) gabapentin (GBP), carbamazepine (CBZ) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) on learning and memory, anxiety, and locomotor activity in rats with lithium-pilocarpine-induced status epilepticus (SE). SE was induced by intraperitoneal injections of 3mEq/kg LiCl followed by 45mg/kg pilocarpine 24h later. In the first series of experiments, rats were divided into four groups three hours after the onset of SE and received GBP (100mug/10mul, two times a day; i.c.v.), CBZ (200mug/10mul; i.c.v.), CNQX (25nmol/10mul; i.c.v.) or saline (10mul; i.c.v.) for 7days. Six weeks after SE, cognitive and behavioral performances were evaluated by Morris water maze, elevated plus maze, and open field tests. In the second series, rats received no treatment for six weeks following SE. On the seventh week the same treatment with the previous rats was given and six weeks later the cognitive and behavioral tests were applied. SE significantly impaired spatial learning and memory in the Morris water maze. GBP treatment improved the acqusition and memory performance. CNQX worsened the acqusition but improved the memory performance, while CBZ worsened both parameters. In the elevated plus maze, epileptic rats which received saline showed significantly lower anxiety levels with respect to the naive rats. Only CBZ led to further anxiolysis, while the other drugs had no effect. Locomotor activity significantly increased due to SE, which was augmented by GBP and CNQX. The impact of immediate and delayed treatment with these drugs on cognition and behavior seems to be quite similar.
Dorsal hippocampus infusions of CNQX into the dentate gyrus disrupt expression of trace fear conditioning.[Pubmed:25565270]
Hippocampus. 2015 Jul;25(7):779-85.
The hippocampus is essential for the consolidation of some explicit long-term memories, including trace conditioning. Lesions and pharmacological manipulations of the dorsal hippocampus (DH) have provided strong evidence for its involvement in the acquisition and expression of trace fear memories. However, no studies have specifically targeted DH subregions [CA1 and dentate gyrus (DG)] to determine their involvement in trace fear conditioning. In the present study, rats received bilateral cannulation targeting either the DG or CA1 of the DH. Following surgery, animals were trace fear conditioned. Forty-eight hours following training, rats received bilateral infusions of the AMPA/kainate glutamate receptor antagonist, CNQX, or vehicle. Following the infusion, rats were placed in a novel context for the tone test. Rats that received CNQX into the DG froze significantly less during the tone and trace interval as compared to controls. Rats that received CNQX into the DH CA1 showed no difference in freezing during the tone or trace interval as compared to controls. These data support a role for the DG in the expression of trace tone fear conditioning.
Antagonism of synaptic potentials in ventral horn neurones by 6-cyano-7-nitroquinoxaline-2,3-dione: a study in the rat spinal cord in vitro.[Pubmed:1358390]
Br J Pharmacol. 1992 Oct;107(2):375-81.
1. The rat spinal cord in vitro has been used to assess the effect of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) on the dorsal root evoked extracellular ventral root reflex (DR-VRR) and the intracellular excitatory postsynaptic potential (e.p.s.p.) in ventral horn neurones and motoneurones. 2. CNQX (1-5 microM) produces a selective and dose-dependent reduction in the amplitude of the monosynaptic component of the DR-VRR recorded from lumbar spinal segments. 3. With low intensity dorsal root stimulation CNQX selectively attenuates the amplitude of the short latency intracellular e.p.s.p. (70% reduction, P < 0.005) and its rise-time (75%, P < 0.01) without affecting the half-time to decay. 4. When high intensity stimulation is used CNQX significantly attenuates the amplitude of the e.p.s.p. (56%, P < 0.005), rise-time (76%, P < 0.01) and abolishes the short latency spike. In addition longer latency synaptic components are attenuated and the half-time to decay significantly reduced (47%, P < 0.005). 5. The results with CNQX are compared to D-aminophosphonovalerate and discussed in relation to the recruitment of low versus high threshold afferents. The data supports an involvement of non-NMDA receptors in transmission through both mono- and polysynaptic pathways in the ventral horn.
Effect of 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline (CNQX) on dorsal root-, NMDA-, kainate- and quisqualate-mediated depolarization of rat motoneurones in vitro.[Pubmed:1976402]
Br J Pharmacol. 1990 Aug;100(4):850-4.
1. Mature in vitro rat spinal cord preparations have been used to compare the depressant effects of 6-cyano-2,3-dihydroxy-7-nitroquinoxalinedione (CNQX) and kynurenate on transmission from low threshold myelinated primary afferents in dorsal roots. EC50 values +/- s.e.mean (number of preparations in parentheses) for depression of the monosynaptic ventral root reflex were respectively 1.0 +/- 0.3 microM (5) and 135 +/- 15 microM (3) for CNQX and kynurenate. Transmission through superior cervical ganglia was not significantly affected by concentrations of CNQX up to 100 microM or kynurenate up to 5 mM. 2. Immature in vitro rat spinal cord preparations were used to measure dose-ratios for antagonism of depolarizations induced by N-methyl-D-aspartate (NMDA), kainate or quisqualate by 4, 10 and 25 microM CNQX. In the presence of 0.75 mM Mg2+ pA2 values +/- s.e.mean were respectively 4.62 +/- 0.05 (16), 5.79 +/- 0.01 (4) and 5.59 +/- 0.05 (16) for each agonist. These values were not significantly altered in the absence of added Mg2+. The mean pA2 values for kainate were significantly higher than those for quisqualate (P less than 0.01). 3. Antagonism of NMDA-induced depolarizations was evident at 10 and 25 but not 4 microM CNQX. The antagonism of NMDA was reversed by D-serine (100 and 200 microM). 4. A similarity between the relative potencies of both CNQX and kynurenate for depression of synaptic transmission and antagonism of amino acid-induced depolarizations indicates that monosynaptic transmission from myelinated primary afferents to motoneurones is mediated by kainate and/or quisqualate sub-types of non-NMDA receptors.
Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists.[Pubmed:2155495]
Trends Pharmacol Sci. 1990 Jan;11(1):25-33.
Development of new selective ligands for excitatory amino acid receptors has been fundamental in supporting this rapidly developing field. Some of the most important ligands have come from the laboratories of Jeff Watkins, Povl Krogsgaard-Larsen and Tage Honore, who collaborate in this double-length review to describe the chemical features and SARs of agonists and antagonists, particularly those features associated with subtype selectivity.
Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists.[Pubmed:2899909]
Science. 1988 Aug 5;241(4866):701-3.
The N-methyl-D-aspartate (NMDA)-subtype of glutamate receptors has been well described as a result of the early appearance of NMDA antagonists, but no potent antagonist for the "non-NMDA" glutamate receptors has been available. Quinoxalinediones have now been found to be potent and competitive antagonists at non-NMDA glutamate receptors. These compounds will be useful in the determination of the structure-activity relations of quisqualate and kainate receptors and the role of such receptors in synaptic transmission in the mammalian brain.


