Bromophenol Bluewidely used in gel loading buffers CAS# 115-39-9 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115-39-9 | SDF | Download SDF |
| PubChem ID | 8272 | Appearance | Powder |
| Formula | C19H10Br4O5S | M.Wt | 669.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in ethanol | ||
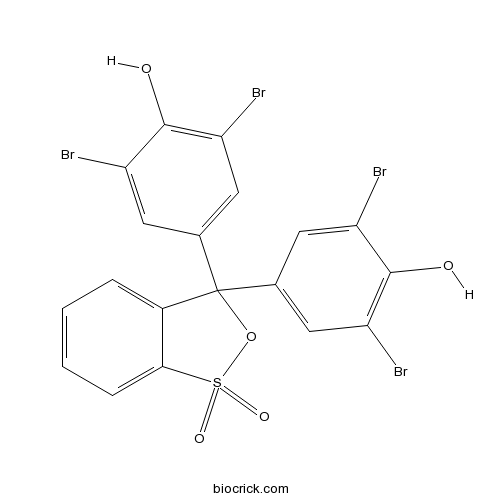
| Chemical Name | 2,6-dibromo-4-[3-(3,5-dibromo-4-hydroxyphenyl)-1,1-dioxo-2,1$l^{6}-benzoxathiol-3-yl]phenol | ||
| SMILES | C1=CC=C2C(=C1)C(OS2(=O)=O)(C3=CC(=C(C(=C3)Br)O)Br)C4=CC(=C(C(=C4)Br)O)Br | ||
| Standard InChIKey | UDSAIICHUKSCKT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H10Br4O5S/c20-12-5-9(6-13(21)17(12)24)19(10-7-14(22)18(25)15(23)8-10)11-3-1-2-4-16(11)29(26,27)28-19/h1-8,24-25H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dye used in gel loading buffers. |

Bromophenol Blue Dilution Calculator

Bromophenol Blue Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4926 mL | 7.4631 mL | 14.9263 mL | 29.8525 mL | 37.3157 mL |
| 5 mM | 0.2985 mL | 1.4926 mL | 2.9853 mL | 5.9705 mL | 7.4631 mL |
| 10 mM | 0.1493 mL | 0.7463 mL | 1.4926 mL | 2.9853 mL | 3.7316 mL |
| 50 mM | 0.0299 mL | 0.1493 mL | 0.2985 mL | 0.5971 mL | 0.7463 mL |
| 100 mM | 0.0149 mL | 0.0746 mL | 0.1493 mL | 0.2985 mL | 0.3732 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
Bromophenol blue (3',3",5',5"-tetrabromophenolsulfonphthalein) is widely used in gel loading buffers. Bromophenol blue is experimentally used as acolor marker, apH indicator, and a dye. Bromophenol blue can be prepared by slowly adding excess bromine to a hot solution ofphenolsulfonphthaleinin the presence of glacial acetic acid.
In vitro: Previous results showed that the adsorption process of bromophenol blue increased with the increase in the concentration of α-chitin nanoparticles (CNP), contact time and temperature, while the adsorption process decreased with increase in the initial dye concentration and strong acidic pH. The Fourier transform infrared spectroscopy results confirmed that the interaction between Bromophenol blue and CNP involved physical adsorption. The adsorption process obeyed Langmuir isotherm and pseudo second order kinetics more effectively. The isotherm and kinetic models confirmed that CNP could be used as a suitable adsorbent material for the removal of dyestuff, such as Bromophenol blue, from effluents [1].
In vivo: So far, bromophenol blue has not been employd in animal in vivo study.
Clinical trial: N/A
Reference:
[1] Dhananasekaran S,Palanivel R,Pappu S. Adsorption of Methylene Blue, Bromophenol Blue, and Coomassie Brilliant Blue by α-chitin nanoparticles. J Adv Res.2016 Jan;7(1):113-24.
- Docetaxel
Catalog No.:BCN5342
CAS No.:114977-28-5
- XL-888
Catalog No.:BCC2339
CAS No.:1149705-71-4
- N1,N10-Bis(p-coumaroyl)spermidine
Catalog No.:BCN6027
CAS No.:114916-05-1
- 2-Chloro-1-(5'-(prop-1-ynyl)-2,2'-bithiophen-5-yl)ethanol
Catalog No.:BCN1614
CAS No.:114916-00-6
- Docetaxel intermediate
Catalog No.:BCN8360
CAS No.:114915-14-9
- Ciwujianoside E
Catalog No.:BCN3505
CAS No.:114912-36-6
- Ciwujianoside B
Catalog No.:BCN1082
CAS No.:114902-16-8
- Z-Val-OH
Catalog No.:BCC2734
CAS No.:1149-26-4
- Trabectedin
Catalog No.:BCC2012
CAS No.:114899-77-3
- Boc-Phe(3-Cl)-OH
Catalog No.:BCC2640
CAS No.:114873-03-9
- Boc-Phe(2-F)-OH
Catalog No.:BCC3223
CAS No.:114873-00-6
- ST-836
Catalog No.:BCC1968
CAS No.:1148156-63-1
- Azacyclonol
Catalog No.:BCC4761
CAS No.:115-46-8
- Sinomenine
Catalog No.:BCN6265
CAS No.:115-53-7
- Ambenonium dichloride
Catalog No.:BCC6630
CAS No.:115-79-7
- Linalyl Acetate
Catalog No.:BCC8200
CAS No.:115-95-7
- 9-Phenylcarbazole
Catalog No.:BCN2259
CAS No.:1150-62-5
- Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3994
CAS No.:115006-86-5
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
Spectrophotometric Determination of Cefixime Trihydrate in Pharmaceutical Formulations Based on Ion-Pair Reaction with Bromophenol Blue.[Pubmed:26279621]
Anal Chem Insights. 2015 Jul 27;10:11-6.
Cefixime trihydrate is a broad spectrum cephalosporin antibiotic, effective against gram-positive and gram-negative bacterial infections. Simple and rapid method has been developed for the determination of cefixime trihydrate in bulk and pharmaceutical formulations. This method was based on the formation of bluish-green ion-pair complex of cefixime trihydrate with Bromophenol Blue in dimethyl sulfoxide (DMSO)-acetonitrile medium. Different parameters were studied and optimized. A 2:1 complex was formed between the drug and reagent almost instantaneously at room temperature which has lambdamax of 610 nm. Under optimum conditions, calibration curve was found to be linear over the range of 10-130 mug mL(-1). The method was subjected to analytical quality control. The limit of detection was found to be 1.08 mug mL(-1). Recovery studies and interference studies were carried out. The proposed method was successfully applied to the determination of cefixime trihydrate in bulk and pharmaceutical formulations with high precision and accuracy.
Origin of the Absorption Band of Bromophenol Blue in Acidic and Basic pH: Insight from a Combined Molecular Dynamics and TD-DFT/MM Study.[Pubmed:27556901]
J Phys Chem A. 2016 Sep 15;120(36):7175-82.
We study the linear and nonlinear optical properties of a well-known acid-base indicator, Bromophenol Blue (BPB), in aqueous solution by employing static and integrated approaches. In the static approach, optical properties have been calculated using time-dependent density functional theory (TD-DFT) on the fully relaxed geometries of the neutral and different unprotonated forms of BPB. Moreover, both closed and open forms of BPB were considered. In the integrated approach, the optical properties have been computed over many snapshots extracted from molecular dynamics simulation using a hybrid time-dependent density functional theory/molecular mechanics approach. The static approach suggests closed neutral --> anionic interconversion as the dominant mechanism for the red shift in the absorption spectra of BPB due to a change from acidic to basic pH. It is found by employing an integrated approach that the two interconversions, namely open neutral --> anionic and open neutral --> dianionic, can contribute to the pH-dependent shift in the absorption spectra of BPB. Even though both static and integrated approaches reproduce the pH-dependent red shift in the absorption spectra of BPB, the latter one is suitable to determine both the spectra and spectral broadening. Finally, the computed static first hyperpolarizability for various protonated and deprotonated forms of BPB reveals that this molecule can be used as a nonlinear optical probe for pH sensing in addition to its highly exploited use as an optical probe.
Adsorption of Methylene Blue, Bromophenol Blue, and Coomassie Brilliant Blue by alpha-chitin nanoparticles.[Pubmed:26843977]
J Adv Res. 2016 Jan;7(1):113-24.
Expelling of dyestuff into water resource system causes major thread to the environment. Adsorption is the cost effective and potential method to remove the dyes from the effluents. Therefore, an attempt was made to study the adsorption of dyestuff (Methylene Blue (MB), Bromophenol Blue (BPB) and Coomassie Brilliant Blue (CBB)) by alpha-chitin nanoparticles (CNP) prepared from Penaeus monodon (Fabricius, 1798) shell waste. On contrary to the most recognizable adsorption studies using chitin, this is the first study using unique nanoparticles of 50 nm used for the dye adsorption process. The results showed that the adsorption process increased with increase in the concentration of CNP, contact time and temperature with the dyestuff, whereas the adsorption process decreased with increase in the initial dye concentration and strong acidic pH. The results from Fourier transform infrared (FTIR) spectroscopy confirmed that the interaction between dyestuff and CNP involved physical adsorption. The adsorption process obeys Langmuir isotherm (R (2) values were 0.992, 0.999 and 0.992 for MB, BPB and CBB, and RL value lies between 0 and 1 for all the three dyes) and pseudo second order kinetics (R (2) values were 0.996, 0.999 and 0.996 for MB, BPB and CBB) more effectively. The isotherm and kinetic models confirmed that CNP can be used as a suitable adsorbent material for the removal of dyestuff from effluents.
Comparative in vitro safety analysis of dyes for chromovitrectomy: indocyanine green, brilliant blue green, bromophenol blue, and infracyanine green.[Pubmed:21394068]
Retina. 2011 Jun;31(6):1128-36.
PURPOSE: Vital dyes such as infracyanine green (IfCG), brilliant blue green (BBG), and Bromophenol Blue (BPB) have been used as an alternative to indocyanine green (ICG) during chromovitrectomy. We compared the in vitro toxicity of IfCG, BBG, and BPB with ICG on the retinal pigment epithelial cells and retinal ganglion cells at various concentrations to optimize the safe dose and duration of exposure. METHODS: Cultured retinal ganglion cells (RGC-5) and human retinal pigment epithelial cells (ARPE-19) were exposed to 2 concentrations (0.25 and 0.5 mg/mL) of ICG, IfCG, BBG, and BPB at various time intervals (1, 5, 15, and 30 minutes). Cell viability was quantified with neutral red assay, and mode of cell death was evaluated with flow cytometry-based Annexin V and propidium iodide staining. RESULTS: Exposure to ICG resulted in 48%-74% reduction in neutral red uptake in both RGC-5 and ARPE-19 cells, after an exposure time of >/=5 minutes compared with control (P < 0.001). Infracyanine green, BBG, and BPB were significantly less toxic on the 2 cell lines at exposure times <15 minutes. (Reduction in cell viability ranged from 6.9% +/- 3.3% to 29.3% +/- 7.4% when compared with control, P > 0.5.) However, among the newer dyes, BBG caused necrosis in retinal pigment epithelial cells and retinal ganglion cells as the exposure time period increased beyond 5 minutes. CONCLUSION: Newer vital dyes, IfCG, BBG, and BPB, are significantly less toxic on retinal ganglion cells and retinal pigment epithelial cells' cell lines when compared with ICG. Infracyanine green was least toxic among the three newer dyes studied.


