DesmethylxanthohumolCAS# 115063-39-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115063-39-3 | SDF | Download SDF |
| PubChem ID | 6443339 | Appearance | Yellow powder |
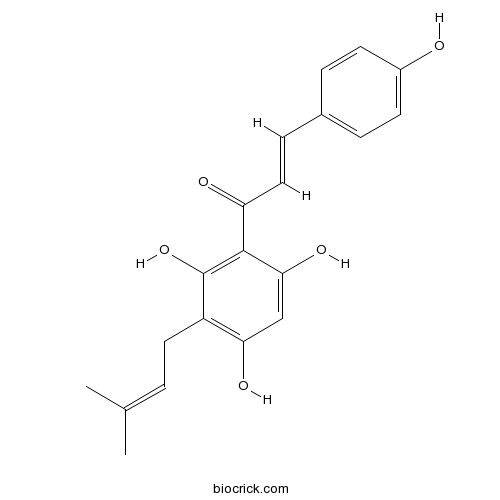
| Formula | C20H20O5 | M.Wt | 340.4 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-hydroxyphenyl)-1-[2,4,6-trihydroxy-3-(3-methylbut-2-enyl)phenyl]prop-2-en-1-one | ||
| SMILES | CC(=CCC1=C(C=C(C(=C1O)C(=O)C=CC2=CC=C(C=C2)O)O)O)C | ||
| Standard InChIKey | FUSADYLVRMROPL-UXBLZVDNSA-N | ||
| Standard InChI | InChI=1S/C20H20O5/c1-12(2)3-9-15-17(23)11-18(24)19(20(15)25)16(22)10-6-13-4-7-14(21)8-5-13/h3-8,10-11,21,23-25H,9H2,1-2H3/b10-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Xanthohumol shows the highest inhibition against cholesteryl ester transfer protein (CETP) from screening of natural products in various plants; prenyl group and chalcone structure of xanthohumol are responsible for the CETP inhibitory activity. |

Desmethylxanthohumol Dilution Calculator

Desmethylxanthohumol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9377 mL | 14.6886 mL | 29.3772 mL | 58.7544 mL | 73.443 mL |
| 5 mM | 0.5875 mL | 2.9377 mL | 5.8754 mL | 11.7509 mL | 14.6886 mL |
| 10 mM | 0.2938 mL | 1.4689 mL | 2.9377 mL | 5.8754 mL | 7.3443 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.1751 mL | 1.4689 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5875 mL | 0.7344 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3994
CAS No.:115006-86-5
- 9-Phenylcarbazole
Catalog No.:BCN2259
CAS No.:1150-62-5
- Linalyl Acetate
Catalog No.:BCC8200
CAS No.:115-95-7
- Ambenonium dichloride
Catalog No.:BCC6630
CAS No.:115-79-7
- Sinomenine
Catalog No.:BCN6265
CAS No.:115-53-7
- Azacyclonol
Catalog No.:BCC4761
CAS No.:115-46-8
- Bromophenol Blue
Catalog No.:BCC8029
CAS No.:115-39-9
- Docetaxel
Catalog No.:BCN5342
CAS No.:114977-28-5
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
Relevance of organic farming and effect of climatological conditions on the formation of alpha-acids, beta-acids, desmethylxanthohumol, and xanthohumol in hop (Humulus lupulus L.).[Pubmed:17199314]
J Agric Food Chem. 2007 Jan 10;55(1):61-6.
The concentrations of alpha-acids, beta-acids, Desmethylxanthohumol, and xanthohumol were monitored in the hop varieties Admiral (A), Wye Challenger (WC), and First Gold (FG) during the harvest seasons of 2003 through 2005. Hops grown under an organic regimen were compared to plants grown conventionally in hop fields in close vicinity. The concentrations of the key compounds depended very much on climatological conditions showing, in general, highest levels in poorest weather conditions (2004). Of the three varieties studied, FG was the only one showing a clear trend for higher concentrations of secondary metabolites under organic growing conditions than under conventional farming conditions. Cultivation of A and WC seems to be very sensitive to climatic conditions and environmental stresses caused by pests and diseases, thereby leading to various results. WC proved to be a rich source of bioactive chalcones, particularly Desmethylxanthohumol.
Synthesis and antioxidant evaluation of desmethylxanthohumol analogs and their dimers.[Pubmed:27688188]
Eur J Med Chem. 2017 Jan 5;125:335-345.
Four ring-closed analogs of natural prenylated chalcone Desmethylxanthohumol (1) and their dimers were synthesized from the commercially available 1-(2,4,6-trihydroxyphenyl)ethan-1-one in five and six linear steps, respectively. The structures of the eight new derivatives were confirmed using(1)H NMR, (13)C NMR and HRMS. The antioxidant activity of the new chalcone derivatives were evaluated in a PC12 cell model of H2O2-induced oxidative damage. The SAR studies suggested that the catechol motif was essential for the antioxidant activity. Moreover, the dimers showed better antioxidant activity than their corresponding monomers did. Among them, compound 14d was the most potent and increased PC12 cell viability from 25% to 85%. Flow cytometric analysis showed that compound 14d, the most potent compound, decreased the apoptotic PC12 cell percentage and significantly reduced the LDH release and 8-OHdG generation but increased the GSH levels in H2O2-treated PC12 cells. Furthermore, compound 14d had a higher FRAP value than that of gallic acid. It also reduced the stable ABTS(+) free radical with a lower EC50 than that of gallic acid.
Dynamic residual complexity of natural products by qHNMR: solution stability of desmethylxanthohumol.[Pubmed:19145555]
Planta Med. 2009 Jun;75(7):757-62.
The use of chromatographic assays to assess the residual complexity of materials that are purified from natural sources by chromatographic means is, in a sense, a case of the fox watching the henhouse. Beside their static residual complexity, which is intrinsic to their metabolic origin, biologically active natural materials can also be involved in chemical reactions that lead to dynamic residual complexity. The present study examines the dynamics of the hop prenylphenol, Desmethylxanthohumol (DMX), by means of quantitative (1)H-NMR (qHNMR) in a setting that mimics IN VITRO and physiological conditions. The experiments provide a comprehensive, time-resolved, and mechanistic picture of the spontaneous isomerization of DMX into congeneric flavanones, including their (1)H/(2)D isotopomers. Formation of the potent phytoestrogen, 8-prenylnaringenin (8PN), suggests that measurable estrogenic activity even of high-purity DMX is an artifact. Together with previously established qHNMR assays including purity activity relationships (PARs), dynamic qHNMR assays complement important steps of the post-isolation evaluation of natural products. Thus, qHNMR allows assessment of several unexpected effects that potentially break the assumed linkage between a single chemical entity (SCE) and biological endpoints.
Formation and accumulation of alpha-acids, beta-acids, desmethylxanthohumol, and xanthohumol during flowering of hops (Humulus lupulus L.).[Pubmed:12848522]
J Agric Food Chem. 2003 Jul 16;51(15):4436-41.
Important secondary metabolites, present in hops (Humulus lupulus L.), include alpha-acids and beta-acids, which are essential for the brewing of beer, as well as the prenylated chalcones, Desmethylxanthohumol, and xanthohumol, which exhibit interesting bioactive properties. Their formation and accumulation in five selected hop varieties, Wye Challenger, Wye Target, Golding, Admiral, and Whitbread Golding Variety, were quantitatively monitored by high-performance liquid chromatography using UV detection. All target compounds were present from the onset of flowering, not only in female hop cones but also in male inflorescences, albeit in low concentrations. During development from female inflorescences to cones, levels of alpha-acids, beta-acids, Desmethylxanthohumol, and xanthohumol gradually increased, while each hop variety exhibited individual accumulation rates. Furthermore, these compounds were present in leaves of fully grown hops as well. The study demonstrated that key compounds for flavor and potential beneficial health effects associated with beer not only reside in the glandular lupulin structures but also are distributed over various parts of the hop plant.


