Cyclo(L-Leu-trans-4-hydroxy-L-Pro)CAS# 115006-86-5 |
- Cyclo(Hpro-Leu)
Catalog No.:BCN2430
CAS No.:1016899-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115006-86-5 | SDF | Download SDF |
| PubChem ID | 21575352 | Appearance | Powder |
| Formula | C11H18N2O3 | M.Wt | 226.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
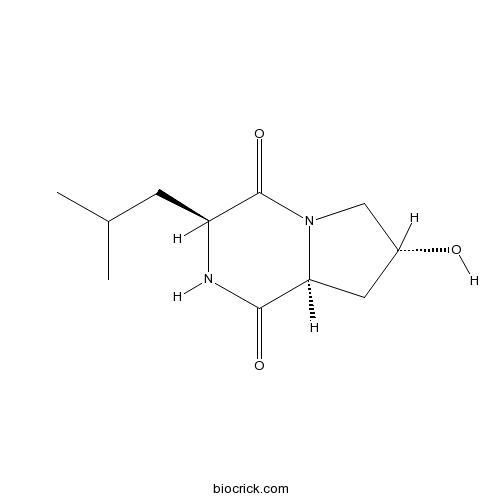
| Chemical Name | (3S,7R,8aS)-7-hydroxy-3-(2-methylpropyl)-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | CC(C)CC1C(=O)N2CC(CC2C(=O)N1)O | ||
| Standard InChIKey | YEHIUWVXPQQDMC-VGMNWLOBSA-N | ||
| Standard InChI | InChI=1S/C11H18N2O3/c1-6(2)3-8-11(16)13-5-7(14)4-9(13)10(15)12-8/h6-9,14H,3-5H2,1-2H3,(H,12,15)/t7-,8+,9+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclo(L-Leu-trans-4-hydroxy-L-Pro) is a natural product from Phellinus igniarius. |
| Structure Identification | Chemical Research & Application, 2014 (9) :1478-1482.Cyclic dipeptides metabolites from the symbiotic fungus paraconiothyrium brasiliense[Reference: WebLink]Nine cyclic dipeptides secondary metabolites were isolated from the symbiotic fungus paraconiothyrium brasiliense,by normal phase silica gel,Sephadex LH-20 chromatography and reverse phase HPLC.
|

Cyclo(L-Leu-trans-4-hydroxy-L-Pro) Dilution Calculator

Cyclo(L-Leu-trans-4-hydroxy-L-Pro) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4189 mL | 22.0946 mL | 44.1891 mL | 88.3783 mL | 110.4728 mL |
| 5 mM | 0.8838 mL | 4.4189 mL | 8.8378 mL | 17.6757 mL | 22.0946 mL |
| 10 mM | 0.4419 mL | 2.2095 mL | 4.4189 mL | 8.8378 mL | 11.0473 mL |
| 50 mM | 0.0884 mL | 0.4419 mL | 0.8838 mL | 1.7676 mL | 2.2095 mL |
| 100 mM | 0.0442 mL | 0.2209 mL | 0.4419 mL | 0.8838 mL | 1.1047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 9-Phenylcarbazole
Catalog No.:BCN2259
CAS No.:1150-62-5
- Linalyl Acetate
Catalog No.:BCC8200
CAS No.:115-95-7
- Ambenonium dichloride
Catalog No.:BCC6630
CAS No.:115-79-7
- Sinomenine
Catalog No.:BCN6265
CAS No.:115-53-7
- Azacyclonol
Catalog No.:BCC4761
CAS No.:115-46-8
- Bromophenol Blue
Catalog No.:BCC8029
CAS No.:115-39-9
- Docetaxel
Catalog No.:BCN5342
CAS No.:114977-28-5
- XL-888
Catalog No.:BCC2339
CAS No.:1149705-71-4
- N1,N10-Bis(p-coumaroyl)spermidine
Catalog No.:BCN6027
CAS No.:114916-05-1
- 2-Chloro-1-(5'-(prop-1-ynyl)-2,2'-bithiophen-5-yl)ethanol
Catalog No.:BCN1614
CAS No.:114916-00-6
- Docetaxel intermediate
Catalog No.:BCN8360
CAS No.:114915-14-9
- Ciwujianoside E
Catalog No.:BCN3505
CAS No.:114912-36-6
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
Streptomyces nigra sp. nov. Is a Novel Actinobacterium Isolated From Mangrove Soil and Exerts a Potent Antitumor Activity in Vitro.[Pubmed:30072967]
Front Microbiol. 2018 Jul 18;9:1587.
A new bacterial strain, designated 452(T), was isolated from the rhizosphere soil of the mangrove Avicennia marina in China. As determined, its cell wall peptidoglycan contained LL-diaminopimelic acid; MK-9(H8) and MK-9(H6) were the major isoprenoid quinones; and iso-C16:0 (31.3%), anteiso-C15:0 (16.9%), and iso-C15:0 (12.5%) were the major cellular fatty acids (>10.0%). Phylogenetic analysis based on the 16S rRNA gene sequence revealed that strain 452(T) formed a distinct lineage in the clade of the genus Streptomyces, and was closely related to S. coerulescens DSM 40146(T) (99.6% sequence identity), S. bellus DSM 40185(T) (99.5%), and S. coeruleorubidus DSM 41172(T) (99.3%). The DNA-DNA relatedness between strain 452(T) and these type strains ranged between 29.3 and 42.3%. Based on the phenotypic, chemotaxonomic, and phylogenetic features, the strain 452(T) is considered to represent a novel species of the genus Streptomyces, for which the name Streptomyces nigra sp. nov. is proposed. The type strain is 452(T) (=KCTC 39960(T) = MCCC 1K03346(T)). Further, strain 452(T) extracts exhibited a pronounced antitumor activity against human cancer cell lines A549, HCT-116, and HepG2, but not against normal human colon cells CCD-18Co. Active substances in the fermentation broth of strain 452(T) were isolated by bioassay-guided analysis, and then purified using a macroporous resin, silica gel, sephadex LX-20 column, and semi-preparative high-performance liquid chromatography (HPLC). Eight proline-containing diketopiperazines, namely, cyclo(Pro-Ala), cyclo(Pro-Gly), cyclo(Pro-Phe), cyclo(Pro-Met), cyclo(Pro-Val), cyclo(Pro-Leu), cyclo(Pro-Tyr), and Cyclo(L-Leu-trans-4-hydroxy-L-Pro), were identified by electrospray ionization mass spectrometry (MS) and nuclear magnetic resonance (NMR). The compounds displayed different levels of cytotoxicity. The highest cytotoxicity was exhibited by cyclo(Pro-Ala) and cyclo(Pro-Met) against A549 cells, and cyclo(Phe-Pro) and cyclo(Pro-Ala) against HCT-116 cells, with average IC50 values equal to 18.5, 27.3, 32.3, and 47.6 mug/mL, respectively. The diversity of diketopiperazines and other chemicals produced by 452(T) was further investigated using gas chromatography (GC)-MS and liquid chromatography (LC)-MS. The analysis revealed 16 types of metabolites with antitumor activity and 16 other types of diketopiperazines. Hence, extracts of the newly identified strain may be used a starting material for the development of antitumor agents.


