XL-888Hsp90 inhibitor CAS# 1149705-71-4 |
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-DMAG (Alvespimycin) HCl
Catalog No.:BCC1175
CAS No.:467214-21-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1149705-71-4 | SDF | Download SDF |
| PubChem ID | 57748689 | Appearance | Powder |
| Formula | C29H37N5O3 | M.Wt | 503.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (198.55 mM) *"≥" means soluble, but saturation unknown. | ||
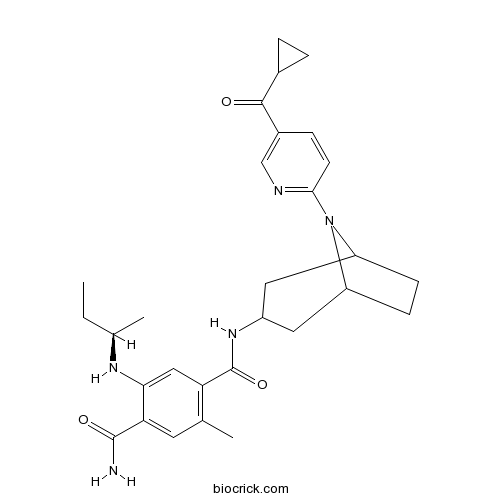
| Chemical Name | 2-[[(2R)-butan-2-yl]amino]-4-N-[8-[5-(cyclopropanecarbonyl)pyridin-2-yl]-8-azabicyclo[3.2.1]octan-3-yl]-5-methylbenzene-1,4-dicarboxamide | ||
| SMILES | CCC(C)NC1=CC(=C(C=C1C(=O)N)C)C(=O)NC2CC3CCC(C2)N3C4=NC=C(C=C4)C(=O)C5CC5 | ||
| Standard InChIKey | LHGWWAFKVCIILM-LAQKFSSHSA-N | ||
| Standard InChI | InChI=1S/C29H37N5O3/c1-4-17(3)32-25-14-23(16(2)11-24(25)28(30)36)29(37)33-20-12-21-8-9-22(13-20)34(21)26-10-7-19(15-31-26)27(35)18-5-6-18/h7,10-11,14-15,17-18,20-22,32H,4-6,8-9,12-13H2,1-3H3,(H2,30,36)(H,33,37)/t17-,20?,21?,22?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | XL-888 is an orally bioavailable, ATP-competitive, small-molecule inhibitor of heat shock protein 90 (Hsp90) with IC50 value of 24 nM. | |||||
| Targets | Hsp90 | |||||
| IC50 | 24 nM | |||||

XL-888 Dilution Calculator

XL-888 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9855 mL | 9.9277 mL | 19.8555 mL | 39.7109 mL | 49.6386 mL |
| 5 mM | 0.3971 mL | 1.9855 mL | 3.9711 mL | 7.9422 mL | 9.9277 mL |
| 10 mM | 0.1986 mL | 0.9928 mL | 1.9855 mL | 3.9711 mL | 4.9639 mL |
| 50 mM | 0.0397 mL | 0.1986 mL | 0.3971 mL | 0.7942 mL | 0.9928 mL |
| 100 mM | 0.0199 mL | 0.0993 mL | 0.1986 mL | 0.3971 mL | 0.4964 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
XL-888 is a novel and orally-bioavailable inhibitor of heat shock protein 90 (HSP90) that selectively inhibits HSP90α and HSP90β with values of 50% inhibition concentration IC50 of 22 nM and 44 nM respectively. It also exerts considerably weaker inhibition against a range of other diverse kinases with IC50 more than 3600 nM for all. X-ray crystallographic analysis reveals that the XL-888 binds to HSP90 through the formation of H-bonding between the N-(R)-sec-butylanthranilamide moiety of XL-888 and ASP93 of HSP90. In recent studies, XL-888 has exhibits strong anti-proliferative activities in a panel of tumor cells with values of IC50 ranging from 0.1 nM to 45.5 nM.
Reference
Paraiso KH, Haarberg HE, Wood E, Rebecca VW, Chen YA, Xiang Y, Ribas A, Lo RS, Weber JS, Sondak VK, John JK, Sarnaik AA, Koomen JM, Smalley KS. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clin Cancer Res. 2012; 18(9):2502-2514
Bussenius J, Blazey CM, Aay N, Anand NK, Arcalas A, Baik T, Bowles OJ, Buhr CA, Costanzo S, Curtis JK, DeFina SC, Dubenko L, Heuer TS, Huang P, Jaeger C, Joshi A, Kennedy AR, Kim AI, Lara K, Lee J, Li J, Lougheed JC, Ma S, Malek S, Manalo JC, Martini JF, McGrath G, Nicoll M, Nuss JM, Pack M, Peto CJ, Tsang TH, Wang L, Womble SW, Yakes M, Zhang W, Rice KD. Discovery of XL888: a novel tropane-derived small molecule inhibitor of HSP90. Bioorg Med Chem Lett. 2012; 22(17): 5396-5404.
- N1,N10-Bis(p-coumaroyl)spermidine
Catalog No.:BCN6027
CAS No.:114916-05-1
- 2-Chloro-1-(5'-(prop-1-ynyl)-2,2'-bithiophen-5-yl)ethanol
Catalog No.:BCN1614
CAS No.:114916-00-6
- Docetaxel intermediate
Catalog No.:BCN8360
CAS No.:114915-14-9
- Ciwujianoside E
Catalog No.:BCN3505
CAS No.:114912-36-6
- Ciwujianoside B
Catalog No.:BCN1082
CAS No.:114902-16-8
- Z-Val-OH
Catalog No.:BCC2734
CAS No.:1149-26-4
- Trabectedin
Catalog No.:BCC2012
CAS No.:114899-77-3
- Boc-Phe(3-Cl)-OH
Catalog No.:BCC2640
CAS No.:114873-03-9
- Boc-Phe(2-F)-OH
Catalog No.:BCC3223
CAS No.:114873-00-6
- ST-836
Catalog No.:BCC1968
CAS No.:1148156-63-1
- Z-Pro-OH
Catalog No.:BCC2754
CAS No.:1148-11-4
- Losartan
Catalog No.:BCC4090
CAS No.:114798-26-4
- Docetaxel
Catalog No.:BCN5342
CAS No.:114977-28-5
- Bromophenol Blue
Catalog No.:BCC8029
CAS No.:115-39-9
- Azacyclonol
Catalog No.:BCC4761
CAS No.:115-46-8
- Sinomenine
Catalog No.:BCN6265
CAS No.:115-53-7
- Ambenonium dichloride
Catalog No.:BCC6630
CAS No.:115-79-7
- Linalyl Acetate
Catalog No.:BCC8200
CAS No.:115-95-7
- 9-Phenylcarbazole
Catalog No.:BCN2259
CAS No.:1150-62-5
- Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3994
CAS No.:115006-86-5
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
Evaluating melanoma drug response and therapeutic escape with quantitative proteomics.[Pubmed:24760959]
Mol Cell Proteomics. 2014 Jul;13(7):1844-54.
The evolution of cancer therapy into complex regimens with multiple drugs requires novel approaches for the development and evaluation of companion biomarkers. Liquid chromatography-multiple reaction monitoring mass spectrometry (LC-MRM) is a versatile platform for biomarker measurement. In this study, we describe the development and use of the LC-MRM platform to study the adaptive signaling responses of melanoma cells to inhibitors of HSP90 (XL888) and MEK (AZD6244). XL888 had good anti-tumor activity against NRAS mutant melanoma cell lines as well as BRAF mutant cells with acquired resistance to BRAF inhibitors both in vitro and in vivo. LC-MRM analysis showed HSP90 inhibition to be associated with decreased expression of multiple receptor tyrosine kinases, modules in the PI3K/AKT/mammalian target of rapamycin pathway, and the MAPK/CDK4 signaling axis in NRAS mutant melanoma cell lines and the inhibition of PI3K/AKT signaling in BRAF mutant melanoma xenografts with acquired vemurafenib resistance. The LC-MRM approach targeting more than 80 cancer signaling proteins was highly sensitive and could be applied to fine needle aspirates from xenografts and clinical melanoma specimens (using 50 mug of total protein). We further showed MEK inhibition to be associated with signaling through the NFkappaB and WNT signaling pathways, as well as increased receptor tyrosine kinase expression and activation. Validation studies identified PDGF receptor beta signaling as a potential escape mechanism from MEK inhibition, which could be overcome through combined use of AZD6244 and the PDGF receptor inhibitor, crenolanib. Together, our studies show LC-MRM to have unique value as a platform for the systems level understanding of the molecular mechanisms of drug response and therapeutic escape. This work provides the proof-of-principle for the future development of LC-MRM assays for monitoring drug responses in the clinic.
Will Hsp90 inhibitors prove effective in BRAF-mutant melanomas?[Pubmed:22442059]
Clin Cancer Res. 2012 May 1;18(9):2420-2.
The RAF inhibitor vemurafenib has unprecedented activity in BRAF-mutant melanomas, but resistance invariably develops. As Hsp90 is required for the stability of several of the oncoproteins that mediate RAF inhibitor resistance, inhibitors of this cellular chaperone may be effective in patients with intrinsic or acquired resistance to RAF inhibitors.
Discovery of XL888: a novel tropane-derived small molecule inhibitor of HSP90.[Pubmed:22877636]
Bioorg Med Chem Lett. 2012 Sep 1;22(17):5396-404.
With structural guidance, tropane-derived HTS hits were modified to optimize for HSP90 inhibition and a desirable in vivo profile. Through an iterative SAR development process 12i (XL888) was discovered and shown to reduce HSP90 client protein content in PD studies. Furthermore, efficacy experiments performed in a NCI-N87 mouse xenograft model demonstrated tumor regression in some dosing regimens.
Inhibition of Wee1, AKT, and CDK4 underlies the efficacy of the HSP90 inhibitor XL888 in an in vivo model of NRAS-mutant melanoma.[Pubmed:23538902]
Mol Cancer Ther. 2013 Jun;12(6):901-12.
The HSP90 inhibitor XL888 is effective at reversing BRAF inhibitor resistance in melanoma, including that mediated through acquired NRAS mutations. The present study has investigated the mechanism of action of XL888 in NRAS-mutant melanoma. Treatment of NRAS-mutant melanoma cell lines with XL888 led to an inhibition of growth, G2-M phase cell-cycle arrest, and the inhibition of cell survival in three-dimensional spheroid and colony formation assays. In vitro, HSP90 inhibition led to the degradation of ARAF, CRAF, Wee1, Chk1, and cdc2 and was associated with decreased mitogen-activated protein kinase (MAPK), AKT, mTOR, and c-jun NH2 kinase (JNK) signaling. Apoptosis induction was associated with increased BIM expression and a decrease in the expression of the prosurvival protein Mcl-1. The critical role of increased BIM and decreased Mcl-1 expression in the survival of NRAS-mutant melanoma cell lines was shown through siRNA knockdown and overexpression studies. In an animal xenograft model of NRAS-mutant melanoma, XL888 treatment led to reduced tumor growth and apoptosis induction. Important differences in the pattern of client degradation were noted between the in vivo and in vitro studies. In vivo, XL888 treatment led to degradation of CDK4 and Wee1 and the inhibition of AKT/S6 signaling with little or no effect observed upon ARAF, CRAF, or MAPK. Blockade of Wee1, using either siRNA knockdown or the inhibitor MK1775, was associated with significant levels of growth inhibition and apoptosis induction. Together, these studies have identified Wee1 as a key target of XL888, suggesting novel therapeutic strategies for NRAS-mutant melanoma.


