Z-Pro-OHCAS# 1148-11-4 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1148-11-4 | SDF | Download SDF |
| PubChem ID | 6927927 | Appearance | Powder |
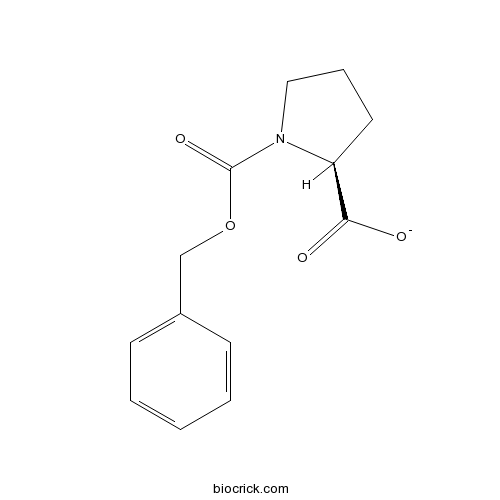
| Formula | C13H15NO4 | M.Wt | 249.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-1-phenylmethoxycarbonylpyrrolidine-2-carboxylate | ||
| SMILES | C1CC(N(C1)C(=O)OCC2=CC=CC=C2)C(=O)[O-] | ||
| Standard InChIKey | JXGVXCZADZNAMJ-NSHDSACASA-M | ||
| Standard InChI | InChI=1S/C13H15NO4/c15-12(16)11-7-4-8-14(11)13(17)18-9-10-5-2-1-3-6-10/h1-3,5-6,11H,4,7-9H2,(H,15,16)/p-1/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Pro-OH Dilution Calculator

Z-Pro-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0112 mL | 20.0562 mL | 40.1123 mL | 80.2246 mL | 100.2808 mL |
| 5 mM | 0.8022 mL | 4.0112 mL | 8.0225 mL | 16.0449 mL | 20.0562 mL |
| 10 mM | 0.4011 mL | 2.0056 mL | 4.0112 mL | 8.0225 mL | 10.0281 mL |
| 50 mM | 0.0802 mL | 0.4011 mL | 0.8022 mL | 1.6045 mL | 2.0056 mL |
| 100 mM | 0.0401 mL | 0.2006 mL | 0.4011 mL | 0.8022 mL | 1.0028 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Pro-OH
- Losartan
Catalog No.:BCC4090
CAS No.:114798-26-4
- 4-Bromomethyl-2-cyanobiphenyl
Catalog No.:BCC8702
CAS No.:114772-54-2
- tert-Butyl4'-(bromomethyl)biphenyl-2-carboxylate
Catalog No.:BCC9164
CAS No.:114772-40-6
- Methyl 4'-bromomethyl biphenyl-2-carboxylate
Catalog No.:BCC9039
CAS No.:114772-38-2
- Methyl 4'-methylbiphenyl-2-carboxylate
Catalog No.:BCC9040
CAS No.:114772-34-8
- 2-(2-Aminobenzoyl)-benzoic acid
Catalog No.:BCC8476
CAS No.:1147-43-9
- Columbianetin
Catalog No.:BCN8502
CAS No.:1147-29-1
- J 147
Catalog No.:BCC6360
CAS No.:1146963-51-0
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- SNS-314 Mesylate
Catalog No.:BCC2177
CAS No.:1146618-41-8
- 3,4-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6490
CAS No.:114637-83-1
- 17alpha-Thevebioside
Catalog No.:BCN6026
CAS No.:114613-59-1
- ST-836
Catalog No.:BCC1968
CAS No.:1148156-63-1
- Boc-Phe(2-F)-OH
Catalog No.:BCC3223
CAS No.:114873-00-6
- Boc-Phe(3-Cl)-OH
Catalog No.:BCC2640
CAS No.:114873-03-9
- Trabectedin
Catalog No.:BCC2012
CAS No.:114899-77-3
- Z-Val-OH
Catalog No.:BCC2734
CAS No.:1149-26-4
- Ciwujianoside B
Catalog No.:BCN1082
CAS No.:114902-16-8
- Ciwujianoside E
Catalog No.:BCN3505
CAS No.:114912-36-6
- Docetaxel intermediate
Catalog No.:BCN8360
CAS No.:114915-14-9
- 2-Chloro-1-(5'-(prop-1-ynyl)-2,2'-bithiophen-5-yl)ethanol
Catalog No.:BCN1614
CAS No.:114916-00-6
- N1,N10-Bis(p-coumaroyl)spermidine
Catalog No.:BCN6027
CAS No.:114916-05-1
- XL-888
Catalog No.:BCC2339
CAS No.:1149705-71-4
- Docetaxel
Catalog No.:BCN5342
CAS No.:114977-28-5
[Synthesis of N-alpha-(arylsulfonyl-L-prolyl)- and N alpha-(benzyloxycarbonyl-L-prolyl)-D,L-4-amidinophenylalanine amides as inhibitors of thrombin].[Pubmed:3725864]
Pharmazie. 1986 Apr;41(4):233-5.
N alpha substituted 4-cyanophenylalanines were prepared by reaction of the acid chloride, the activated ester and the mixed anhydride of Tos-Pro-OH, 2-naphthylsulfonyl-L-proline and Z-Pro-OH, respectively, with cyanophenylalanine. These acids were transferred into the amides via the 4-nitrophenyl esters or the mixed anhydrides. The cyano compounds were converted via the thioamides and the thioimidic esters into the amidines by common way. The exchange of the glycine residue by prolin in compounds former described caused a decreased antithrombin effect.
Thermolysin and alpha-chymotrypsin mediated synthesis of tripeptides containing proline.[Pubmed:3366545]
Int J Pept Protein Res. 1988 Feb;31(2):116-25.
The tripeptides Z-Pro-Leu-Gly-OEt, Z-Pro-Leu-Gly-NH2 and Z-Pro-Leu-Gly-OBzl were synthesized by thermolysin and alpha-chymotrypsin catalysis. The optimum conditions for the couplings between Z-Pro-OH and H-Leu-OEt or H-Leu-Gly-OEt catalysed by thermolysin were determined by a systematic study involving analysis of pH effect, ammonium sulfate concentration, reaction time, enzyme concentration, and relative proportion of the carboxyl and amine components. The best yield obtained for Z-Pro-Leu-OEt was 77% and for Z-Pro-Leu-Gly-OEt, 100%. Z-Pro-Leu-OEt was coupled to H-Gly-OEt, H-Gly-NH2 and H-Gly-OBzl. The best conditions to obtain Z-Pro-Leu-Gly-OEt and Z-Pro-Leu-Gly-NH2 were determined by the study of some factors that affect the reaction yield, such as organic solvent presence, substrate ratio and aqueous and organic solvent ratio. The yield obtained under optimum synthesis conditions was 55% for Z-Pro-Leu-Gly-OEt and 61% for Z-Pro-Leu-Gly-NH2. Z-Pro-Leu-Gly-OBzl was synthesized with 42% yield.
Synthesis of modified tuftsins containing monosaccharides or monosaccharide derivatives.[Pubmed:3570665]
Int J Pept Protein Res. 1987 Feb;29(2):250-61.
Synthesis of some modified tuftsins is described in which a monosaccharide or a monosaccharide derivative was incorporated in the molecule. Acylation of H-Thr-Lys(Z)-Pro-Arg(NO2)-OBzl with D(+)-gluco-1,5-lactone followed by catalytic hydrogenation gave N alpha-gluconyl-tuftsin. Glycosylation of the carboxyl function of the C-terminal arginine has been achieved by reacting, through the mixed anhydride procedure, Boc-Thr-Lys(Z)-Pro-OH with 2-deoxy-2-(NG-nitroargininamido)-D-glucopyranose followed by catalytic hydrogenation and trifluoroacetic acid treatment. O-Glucosyl-tuftsin has been prepared by reacting o-nitrophenyl N-benzyloxycarbonyl-O-[(alpha + beta) 2,3,4,6-tetra-O-benzyl-D-glucopyranosyl]-threoninate with H-Lys(Z)-Pro-Arg(NO2)-OBzl in the presence of 1-hydroxybenzotriazole. Flash chromatography on silica gel allowed a partial separation of the diastereoisomers, one of which has been isolated in a reasonable yield. The single diastereoisomer and the alpha + beta anomeric mixture were separately deblocked by catalytic hydrogenation and purified by RP-HPLC.
Synthesis, radiolabeling and biological activity of peptide oostatic hormone and its analogues.[Pubmed:9309578]
J Pept Res. 1997 Sep;50(3):153-8.
A series of Pro peptides containing the sequence of the oostatic hormone 3d and its shorter analogues 3a-3c differing in a number of the C-terminal Pro residues was prepared for a study of its effect on oogenesis in Sarcophaga bullata Parker (Diptera). Peptides 3a-3d were synthesized in solution by the fragment condensation of Boc-Tyr-Asp(OtBu)-Pro-Ala-Pro-OH (2f) with Pro oligopeptides H-(Pro)2-5-OtBu. The amino-terminal protected pentapeptide acid 2f was prepared by a stepwise procedure from TFA.H-Ala-Pro-OMe using Boc-Pro-OH, Z-Asp(OtBu)-OSu and Boc-Tyr-OSu. The H(Z)-(Pro)2-5-OtBu oligopeptides 1a-1h were synthesized from Z-Pro-OH and H-Pro-OtBu by a combination of stepwise procedure and fragment condensation. The 125I-labeled molecules of the octapeptide 3b and decapeptide 3d were used for radiotracer distribution studies. Evidence of content of the labeled peptide material in various parts of the insect body (ovaries, head, intestine) is presented. The time distribution of the labeled material in the insect organs was correlated with results of histological analysis of ovaries treated by nonlabeled peptides. The peptides assayed affected processes of egg development in 20-60% of ovarioles. The decapeptide 3d caused changes consisting in some resorbed egg chambers and normal appearance of vitellogenic eggs, whereas the octapeptide 3b caused abnormal yolk deposition and formation of big eggs with irregular yolk granules, proliferation of follicular epithelium in some egg chambers and about the same amount of resorbed egg chambers as decapeptide. These structural differences are complementary to the different values of organ radioactivities.


