ColumbianetinCAS# 1147-29-1 |
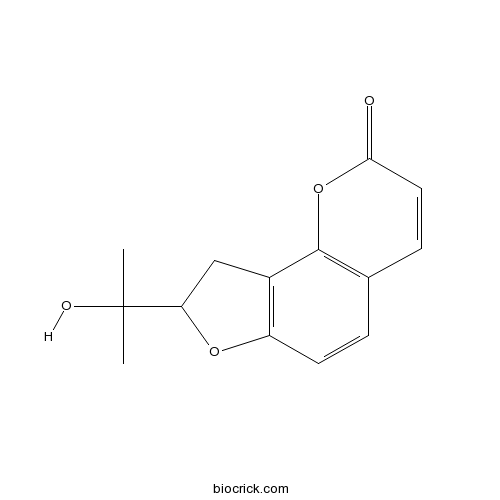
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1147-29-1 | SDF | Download SDF |
| PubChem ID | 150888 | Appearance | White granular crystal |
| Formula | C14H14O4 | M.Wt | 246.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 8-(2-hydroxypropan-2-yl)-8,9-dihydrofuro[2,3-h]chromen-2-one | ||
| SMILES | CC(C)(C1CC2=C(O1)C=CC3=C2OC(=O)C=C3)O | ||
| Standard InChIKey | YRAQEMCYCSSHJG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14O4/c1-14(2,16)11-7-9-10(17-11)5-3-8-4-6-12(15)18-13(8)9/h3-6,11,16H,7H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Columbianetin Dilution Calculator

Columbianetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0607 mL | 20.3037 mL | 40.6075 mL | 81.215 mL | 101.5187 mL |
| 5 mM | 0.8121 mL | 4.0607 mL | 8.1215 mL | 16.243 mL | 20.3037 mL |
| 10 mM | 0.4061 mL | 2.0304 mL | 4.0607 mL | 8.1215 mL | 10.1519 mL |
| 50 mM | 0.0812 mL | 0.4061 mL | 0.8121 mL | 1.6243 mL | 2.0304 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.4061 mL | 0.8121 mL | 1.0152 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- J 147
Catalog No.:BCC6360
CAS No.:1146963-51-0
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- SNS-314 Mesylate
Catalog No.:BCC2177
CAS No.:1146618-41-8
- 3,4-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6490
CAS No.:114637-83-1
- 17alpha-Thevebioside
Catalog No.:BCN6026
CAS No.:114613-59-1
- Moracin T
Catalog No.:BCN3291
CAS No.:1146113-27-0
- Kisspeptin 234
Catalog No.:BCC6077
CAS No.:1145998-81-7
- Soyasaponin Ba
Catalog No.:BCN2854
CAS No.:114590-20-4
- Thevebioside
Catalog No.:BCN6025
CAS No.:114586-47-9
- Coronadiene
Catalog No.:BCN3683
CAS No.:1145689-64-0
- Ganoderiol F
Catalog No.:BCN6024
CAS No.:114567-47-4
- Boeravinone B
Catalog No.:BCN6466
CAS No.:114567-34-9
- 2-(2-Aminobenzoyl)-benzoic acid
Catalog No.:BCC8476
CAS No.:1147-43-9
- Methyl 4'-methylbiphenyl-2-carboxylate
Catalog No.:BCC9040
CAS No.:114772-34-8
- Methyl 4'-bromomethyl biphenyl-2-carboxylate
Catalog No.:BCC9039
CAS No.:114772-38-2
- tert-Butyl4'-(bromomethyl)biphenyl-2-carboxylate
Catalog No.:BCC9164
CAS No.:114772-40-6
- 4-Bromomethyl-2-cyanobiphenyl
Catalog No.:BCC8702
CAS No.:114772-54-2
- Losartan
Catalog No.:BCC4090
CAS No.:114798-26-4
- Z-Pro-OH
Catalog No.:BCC2754
CAS No.:1148-11-4
- ST-836
Catalog No.:BCC1968
CAS No.:1148156-63-1
- Boc-Phe(2-F)-OH
Catalog No.:BCC3223
CAS No.:114873-00-6
- Boc-Phe(3-Cl)-OH
Catalog No.:BCC2640
CAS No.:114873-03-9
- Trabectedin
Catalog No.:BCC2012
CAS No.:114899-77-3
- Z-Val-OH
Catalog No.:BCC2734
CAS No.:1149-26-4
Simultaneous assessment of absorption characteristics of coumarins from Angelicae Pubescentis Radix: In vitro transport across Caco-2 cell and in vivo pharmacokinetics in rats after oral administration.[Pubmed:28654868]
J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Aug 15;1060:308-315.
Angelicae Pubescentis Radix (APR), a well-known traditional Chinese medicine, is widely used for the treatments of rheumatism and headache for centuries. To assess the absorption characteristics of coumarins from APR, a sensitive and reliable UPLC-MS/MS method was established for the simultaneous determination of sixteen coumarins from APR, including psoralen, xanthotoxin, bergapten, bergaptol, isoimperatorin, imperatorin, Columbianetin, Columbianetin acetate, columbianadin, oxypeucedanin hydrate, angelol B, umbelliferone, scopoletin, osthole, meranzin hydrate and nodakenetin. The specificity, linearity, sensitivity, precision, accuracy, recovery, matrix effect and stability of the method were all validated to be satisfactory. The method was then applied to the in vitro transport of APR extract (APRE) across human intestinal epithelial Caco-2 cell and in vivo pharmacokinetics in rats after oral administration of APRE. All of the tested coumarins were well or moderately absorbed across Caco-2 monolayers, and could be quickly absorbed into rat blood circulation after oral administration. Columbianetin was the most easily absorbed compound across Caco-2 cell, and also had extremely highest plasma concentration in vivo. Excellent correlation between in vitro absorption across Caco-2 cell monolayers and in vivo pharmacokinetics of coumarins from APRE was well verified. The results provided valuable information for the overall absorption characteristics of the coumarins from APR, as well as for its further studies of in vivo active substances in the further.
Simultaneous determination of columbianetin-beta-d-glucopyranoside and columbianetin in a biological sample by high-performance liquid chromatography with fluorescence detection and identification of other columbianetin-beta-d-glucopyranoside metabolites by ultra high-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry.[Pubmed:29506005]
J Pharm Biomed Anal. 2018 May 10;153:221-231.
Columbianetin-beta-d-glucopyranoside (CBG) and its metabolite Columbianetin (CBN) are the bioactive constituents of Angelicae pubescentis radix (APR). They exhibit the anti-platelet aggregation, anti-inflammatory and analgesic properties. The absorption, distribution, metabolism and excretion (ADME) of CBG has not been reported to date. Both high-performance liquid chromatography with fluorescence detection and ultra high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry methods were developed and validated for the study of ADME of CBG. It was found that CBG could be catabolized into its active metabolite CBN in vivo. The absolute bioavailability of Columbianetin-beta-d-glucopyranoside was 5.63+/-4.42%. The other co-existing constituents from the APR ethanol extract could enhance the absorption of CBG. CBG and CBN were rapidly and broadly distributed in the stomach, ovary, kidney, liver, spleen, lung, muscles, heart and brain. Higher levels of accumulation of CBG and CBN were detected in the ovary and kidney tissues. Eight metabolites of CBG were tentatively identified in blood, urine, bile and faeces of rats after oral administration of pure CBG. It was also found that CBG and CBN were mainly excreted through the faecal route. It can be concluded that the validated methods were successfully applied for absorption, distribution, metabolism and excretion study of CBG.
Distribution Assessments of Coumarins from Angelicae Pubescentis Radix in Rat Cerebrospinal Fluid and Brain by Liquid Chromatography Tandem Mass Spectrometry Analysis.[Pubmed:29361720]
Molecules. 2018 Jan 20;23(1). pii: molecules23010225.
Angelicae Pubescentis Radix (APR) is a widely-used traditional Chinese medicine. Pharmacological studies have begun to probe its biological activities on neurological disorders recently. To assess the brain penetration and distribution of APR, a validated ultra-performance liquid chromatography tandem mass spectrometry method was applied to the simultaneous determinations of the main coumarins from APR in the rat cerebrospinal fluid (CSF) and brain after oral administration of APR extract, including psoralen, xanthotoxin, bergapten, isoimperatorin, Columbianetin, Columbianetin acetate, columbianadin, oxypeucedanin hydrate, angelol B, osthole, meranzin hydrate and nodakenetin. Most of the tested coumarins entered the rat CSF and brain quickly, and double-peak phenomena in concentration-time curves were similar to those of their plasma pharmacokinetics. Columbianetin had the highest concentration in the CSF and brain, while psoralen and Columbianetin acetate had the largest percent of CSF/plasma and brain/plasma, indicating that these three coumarins may be worthy of further research on the possible nervous effects. Correlations between the in vivo brain distributions and plasma pharmacokinetics of these coumarins were well verified. These results provided valuable information for the overall in vivo brain distribution characteristics of APR and also for its further studies on the active substances for the central nervous system.
Identification of Nematicidal Constituents of Notopterygium incisum Rhizomes against Bursaphelenchus xylophilus and Meloidogyne incognita.[Pubmed:27669203]
Molecules. 2016 Sep 23;21(10). pii: molecules21101276.
During a screening program for new agrochemicals from Chinese medicinal herbs, the ethanol extract of Notopterygium incisum rhizomes was found to possess strong nematicidal activity against the two species of nematodes, Bursaphelenchus xylophilus and Meloidogyne incognita. Based on bioactivity-guided fractionation, the four constituents were isolated from the ethanol extract and identified as Columbianetin, falcarindiol, falcarinol, and isoimperatorin. Among the four isolated constituents, two acetylenic compounds, falcarindiol and falcarinol (2.20-12.60 mug/mL and 1.06-4.96 mug/mL, respectively) exhibited stronger nematicidal activity than two furanocoumarins, Columbianetin, and isoimperatorin (21.83-103.44 mug/mL and 17.21-30.91 mug/mL, respectively) against the two species of nematodes, B. xylophilus and M. incognita. The four isolated constituents also displayed phototoxic activity against the nematodes. The results indicate that the ethanol extract of N. incisum and its four isolated constituents have potential for development into natural nematicides for control of plant-parasitic nematodes.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica cv.Yubaizhi].[Pubmed:28822155]
Zhongguo Zhong Yao Za Zhi. 2017 Jun;42(11):2102-2109.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica cv. Yubaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Thirty-three compounds were obtained and identified as isoimperatorin (1), imperatorin (2), stigmasterol (3), isooxypeucedanin (4), pabulenol (5), psoralen (6), bergapten (7), isodemethylfuropinarine (8), phellopterin (9), osthenol (10), alloimperatorin (11), xanthotoxin (12), xanthotoxol (13), isopimpinellin (14), alloisoimperatorin (15), beta-sitosterol (16), oxyalloimperatorin (17), pabularinone (18), 5-hydroxy-8-methoxypsoralen (19), Columbianetin (20), heracol (21), isogosferol (22), 2''R-neobyakangelicol (23), byakangelicin ethoxide (24), byakangelicin (25), oxypeucedanin hydrate (26), uracil (27), umbelliferone (28), bergaptol (29), demethylfuropinarine (30), isobyakangelicol (31), oxypeucedanin ethanolate (32), heraclenol (33). Among them, compounds 8, 10, 17, 21, and 30 were obtained from the roots of title plant for the first time.


