Ganoderiol FCAS# 114567-47-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114567-47-4 | SDF | Download SDF |
| PubChem ID | 471008 | Appearance | Powder |
| Formula | C30H46O3 | M.Wt | 454.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
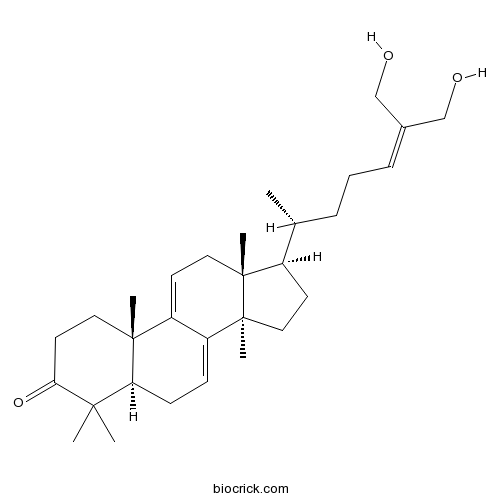
| Chemical Name | (5R,10S,13R,14R,17R)-17-[(2R)-7-hydroxy-6-(hydroxymethyl)hept-5-en-2-yl]-4,4,10,13,14-pentamethyl-1,2,5,6,12,15,16,17-octahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CC(CCC=C(CO)CO)C1CCC2(C1(CC=C3C2=CCC4C3(CCC(=O)C4(C)C)C)C)C | ||
| Standard InChIKey | JVGJXXNUVVQEIG-MCKXIFHVSA-N | ||
| Standard InChI | InChI=1S/C30H46O3/c1-20(8-7-9-21(18-31)19-32)22-12-16-30(6)24-10-11-25-27(2,3)26(33)14-15-28(25,4)23(24)13-17-29(22,30)5/h9-10,13,20,22,25,31-32H,7-8,11-12,14-19H2,1-6H3/t20-,22-,25+,28-,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ganoderiol F has anti-inflammatory, cytotoxic and anti-HIV activity, it inhibits activity of topoisomerases in vitro, and it inhibits human immunodeficiency virus-1 protease with IC(50) values of 20-40 microM. It induced HO-1 expression, activation of the mitogen-activated protein kinase EKR and up-regulation of cyclin-dependent kinase inhibitor p16 and suppressed lipopolysaccharide (LPS)-induced nitric oxide (NO) production. |
| Targets | Androgen Receptor | HIV | NO | HO-1 |
| In vitro | Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells.[Pubmed: 25239868]Toxicol Appl Pharmacol. 2014 Nov 1;280(3):434-42.Ganoderma lucidum is a popular medicinal mushroom used in traditional medicine for preventing or treating a variety of diseases.
|
| In vivo | Anti-human immunodeficiency virus-1 protease activity of new lanostane-type triterpenoids from Ganoderma sinense.[Pubmed: 19801861]Chem Pharm Bull (Tokyo). 2009 Oct;57(10):1076-80.Five new highly oxygenated lanostane-type triterpenoids [ganoderic acid GS-1 (1), ganoderic acid GS-2 (2), ganoderic acid GS-3 (3), 20(21)-dehydrolucidenic acid N (4) and 20-hydroxylucidenic acid A (5)] were isolated from the fruiting body of Ganoderma sinense, together with known compounds including 6 triterpenoids and 3 sterols.
|
| Cell Research | Ganoderiol F, a ganoderma triterpene, induces senescence in hepatoma HepG2 cells.[Pubmed: 16635496]Life Sci. 2006 Aug 15;79(12):1129-39.Ganoderiol F (GolF), a tetracyclic triterpene, was isolated from Ganoderma amboinense and found to induce senescence of cancer cell lines.
|

Ganoderiol F Dilution Calculator

Ganoderiol F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1993 mL | 10.9963 mL | 21.9925 mL | 43.985 mL | 54.9813 mL |
| 5 mM | 0.4399 mL | 2.1993 mL | 4.3985 mL | 8.797 mL | 10.9963 mL |
| 10 mM | 0.2199 mL | 1.0996 mL | 2.1993 mL | 4.3985 mL | 5.4981 mL |
| 50 mM | 0.044 mL | 0.2199 mL | 0.4399 mL | 0.8797 mL | 1.0996 mL |
| 100 mM | 0.022 mL | 0.11 mL | 0.2199 mL | 0.4399 mL | 0.5498 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boeravinone B
Catalog No.:BCN6466
CAS No.:114567-34-9
- Galanin (1-29) (rat, mouse)
Catalog No.:BCC5928
CAS No.:114547-31-8
- Regelidine
Catalog No.:BCN3094
CAS No.:114542-54-0
- Honyucitrin
Catalog No.:BCN4728
CAS No.:114542-44-8
- Spiramine A
Catalog No.:BCN6023
CAS No.:114531-28-1
- (+)-U-50488 hydrochloride
Catalog No.:BCC6656
CAS No.:114528-81-3
- (-)-U-50488 hydrochloride
Catalog No.:BCC6666
CAS No.:114528-79-9
- Z-Ser-OH
Catalog No.:BCC2743
CAS No.:1145-80-8
- Oroxin B
Catalog No.:BCN1203
CAS No.:114482-86-9
- BNP (1-32), human
Catalog No.:BCC1039
CAS No.:114471-18-0
- Beta-Furoyleupatolide
Catalog No.:BCN6407
CAS No.:114437-24-0
- WYE-125132 (WYE-132)
Catalog No.:BCC4608
CAS No.:1144068-46-1
- Coronadiene
Catalog No.:BCN3683
CAS No.:1145689-64-0
- Thevebioside
Catalog No.:BCN6025
CAS No.:114586-47-9
- Soyasaponin Ba
Catalog No.:BCN2854
CAS No.:114590-20-4
- Kisspeptin 234
Catalog No.:BCC6077
CAS No.:1145998-81-7
- Moracin T
Catalog No.:BCN3291
CAS No.:1146113-27-0
- 17alpha-Thevebioside
Catalog No.:BCN6026
CAS No.:114613-59-1
- 3,4-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6490
CAS No.:114637-83-1
- SNS-314 Mesylate
Catalog No.:BCC2177
CAS No.:1146618-41-8
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- J 147
Catalog No.:BCC6360
CAS No.:1146963-51-0
- Columbianetin
Catalog No.:BCN8502
CAS No.:1147-29-1
- 2-(2-Aminobenzoyl)-benzoic acid
Catalog No.:BCC8476
CAS No.:1147-43-9
Anti-human immunodeficiency virus-1 protease activity of new lanostane-type triterpenoids from Ganoderma sinense.[Pubmed:19801861]
Chem Pharm Bull (Tokyo). 2009 Oct;57(10):1076-80.
Five new highly oxygenated lanostane-type triterpenoids [ganoderic acid GS-1 (1), ganoderic acid GS-2 (2), ganoderic acid GS-3 (3), 20(21)-dehydrolucidenic acid N (4) and 20-hydroxylucidenic acid A (5)] were isolated from the fruiting body of Ganoderma sinense, together with known compounds including 6 triterpenoids and 3 sterols. The structures of the new triterpenoids determined by spectroscopic means including 2D NMR were 7beta-hydroxy-3,11,15-trioxo-lanosta-8,24(E)-dien-26-oic acid (1), 7beta,15alpha-dihydroxy-3,11-dioxo-lanosta-8,24(E)-dien-26-oic acid (2), 12beta-acetoxy-3beta,7beta-dihydroxy-11,15-dioxo-lanosta-8,24(E)-dien-26-oic acid (3), 3beta,7beta-dihydroxy-11,15-dioxo-25,26,27-trinorlanosta-8,20-dien-24-oic acid (4), and 7beta,20xi-dihydroxy-3,11,15-trioxo-25,26,27-trinorlanost-8-en-24-oic acid (5), respectively. Among these, ganoderic acid GS-2, 20-hydroxylucidenic acid N, 20(21)-dehydrolucidenic acid N and Ganoderiol F inhibited human immunodeficiency virus-1 protease with IC(50) values of 20-40 microM.
Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells.[Pubmed:25239868]
Toxicol Appl Pharmacol. 2014 Nov 1;280(3):434-42.
Ganoderma lucidum is a popular medicinal mushroom used in traditional medicine for preventing or treating a variety of diseases. In the present study, we investigated the anti-inflammatory and heme oxygenase (HO)-1 inducing effects of 12 lanostane triterpenes from G. lucidum in RAW264.7 cells. Of these, seven triterpenes, butyl lucidenateE2, butyl lucidenateD2 (GT-2), butyl lucidenate P, butyl lucidenateQ, Ganoderiol F, methyl ganodenate J and butyl lucidenate N induced HO-1 expression and suppressed lipopolysaccharide (LPS)-induced nitric oxide (NO) production. Inhibiting HO-1 activity abrogated the inhibitory effects of these triterpenes on the production of NO in LPS-stimulated RAW264.7 cells, suggesting the involvement of HO-1 in the anti-inflammatory effects of these triterpenes. We further studied the anti-inflammatory and HO-1 inducing effects of GT-2. Mitogen-activated protein kinase inhibitors or N-acetylcysteine, an antioxidant, did not suppress GT-2-mediated HO-1 induction; however, LY294002, a phosphoinositide 3-kinase (PI3K) inhibitor, blocked GT-2-induced HO-1 mRNA and protein expression. GT-2 increased nuclear translocation of nuclear factor-E2-related factor 2 (Nrf2) and knockdown of Nrf2 by small interfering RNA blocked GT-2-mediated HO-1 induction, suggesting that GT-2 induced HO-1 expression via the PI3K/AKT-Nrf2 pathway. Consistent with the notion that HO-1 has anti-inflammatory properties, GT-2 inhibited the production of tumor necrosis factor-alpha and interleukin-6, as well as inducible nitric oxide synthase and cyclooxygenase-2 expression. These findings suggest that HO-1 inducing activities of these lanostane triterpenes may be important in the understanding of a novel mechanism for the anti-inflammatory activity of G. lucidum.


