BNP (1-32), humanBrain natriuretic peptide CAS# 114471-18-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114471-18-0 | SDF | Download SDF |
| PubChem ID | 53325981 | Appearance | Powder |
| Formula | C143H244N50O42S4 | M.Wt | 3464.04 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Brain natriuretic peptide (1-32) (human);BNP-32 (human) | ||
| Solubility | >206.6mg/ml in DMSO | ||
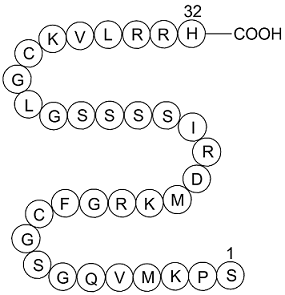
| Sequence | H2N-Ser-Pro-Lys-Met-Val-Gln-Gly-Ser-Gly-Cys-Phe-Gly-Arg-Lys-Met-Asp-Arg-Ile-Ser-Ser-Ser-Ser-Gly-Leu-Gly-Cys-Lys-Val-Leu-Arg-Arg-His-OH | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(4R,10S,16S,19S,22S,25S,28S,31S,34S,37S,40S,43S,49S,52R)-52-[[2-[[(2S)-2-[[2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-1-[(2S)-2-amino-3-hydroxypropanoyl]pyrrolidine-2-carbonyl]amino]hexanoyl]amino]-4-methylsulfanylbutanoyl]amino]-3-methylbutanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-40-(4-aminobutyl)-49-benzyl-28-[(2S)-butan-2-yl]-31,43-bis(3-carbamimidamidopropyl)-34-(carboxymethyl)-16,19,22,25-tetrakis(hydroxymethyl)-10-(2-methylpropyl)-37-(2-methylsulfanylethyl)-6,9,12,15,18,21,24,27,30,33,36,39,42,45,48,51-hexadecaoxo-1,2-dithia-5,8,11,14,17,20,23,26,29,32,35,38,41,44,47,50-hexadecazacyclotripentacontane-4-carbonyl]amino]hexanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-(1H-imidazol-5-yl)propanoic acid | ||
| SMILES | CCC(C)C1C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NCC(=O)NC(CSSCC(C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCNC(=N)N)CC(=O)O)CCSC)CCCCN)CCCNC(=N)N)CC2=CC=CC=C2)NC(=O)CNC(=O)C(CO)NC(=O)CNC(=O)C(CCC(=O)N)NC(=O)C(C(C)C)NC(=O)C(CCSC)NC(=O)C(CCCCN)NC(=O)C3CCCN3C(=O)C(CO)N)C(=O)NC(CCCCN)C(=O)NC(C(C)C)C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC4=CN=CN4)C(=O)O)CC(C)C)CO)CO)CO)CO | ||
| Standard InChIKey | HPNRHPKXQZSDFX-OAQDCNSJSA-N | ||
| Standard InChI | InChI=1S/C143H244N50O42S4/c1-13-76(10)112-137(232)189-99(68-199)131(226)188-98(67-198)130(225)187-97(66-197)129(224)186-96(65-196)117(212)166-59-105(202)169-90(52-72(2)3)114(209)163-61-107(204)171-100(132(227)177-83(32-19-22-44-146)124(219)190-111(75(8)9)136(231)184-91(53-73(4)5)127(222)175-84(34-24-46-159-141(151)152)121(216)174-85(35-25-47-160-142(153)154)122(217)185-94(139(234)235)55-78-57-157-71-167-78)69-238-239-70-101(172-108(205)62-165-116(211)95(64-195)170-106(203)60-162-113(208)87(38-39-103(148)200)181-135(230)110(74(6)7)191-126(221)89(41-51-237-12)179-120(215)82(31-18-21-43-145)180-134(229)102-37-27-49-193(102)138(233)79(147)63-194)133(228)182-92(54-77-28-15-14-16-29-77)115(210)164-58-104(201)168-80(33-23-45-158-140(149)150)118(213)173-81(30-17-20-42-144)119(214)178-88(40-50-236-11)123(218)183-93(56-109(206)207)128(223)176-86(125(220)192-112)36-26-48-161-143(155)156/h14-16,28-29,57,71-76,79-102,110-112,194-199H,13,17-27,30-56,58-70,144-147H2,1-12H3,(H2,148,200)(H,157,167)(H,162,208)(H,163,209)(H,164,210)(H,165,211)(H,166,212)(H,168,201)(H,169,202)(H,170,203)(H,171,204)(H,172,205)(H,173,213)(H,174,216)(H,175,222)(H,176,223)(H,177,227)(H,178,214)(H,179,215)(H,180,229)(H,181,230)(H,182,228)(H,183,218)(H,184,231)(H,185,217)(H,186,224)(H,187,225)(H,188,226)(H,189,232)(H,190,219)(H,191,221)(H,192,220)(H,206,207)(H,234,235)(H4,149,150,158)(H4,151,152,159)(H4,153,154,160)(H4,155,156,161)/t76-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,110-,111-,112-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Cell experiment: [1] | |
| Cell lines | Normal adult canine ventricular fibroblasts |
| Preparation method | The solubility of this peptide in sterile water is >10 mM. Stock solution should be splited and stored at -80°C for several months. |
| Reacting condition | 1 μM, 5 min |
| Applications | BNP (1 μM) significantly increased the intracellular cGMP, whereas lower concentrations did not alter the cGMP. At this concentration, BNP elevated cGMP levels with a maximal effect at 5 minutes. BNP (1 μM) significantly inhibited the [3H]proline incorporation into the cells by 29%. |
| Animal experiment: [2] | |
| Animal models | Adult Japanese white rabbits |
| Dosage form | Injected through the sclera into the vitreous cavity, 100 μM, 4 hours |
| Application | At 100 μM, BNP treatment induced a significant decrease in IOP compared with vehicle-treated eyes. In particular, there were statistically significant differences at 4 and 6 hours. In addition, BNP treatment at 10 μM caused a significant decrease in IOP compared to the vehicle-treated eyes, but only at 6 hours after the injection. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Tsuruda T, Boerrigter G, Huntley B K, et al. Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circulation research, 2002, 91(12): 1127-1134. [2] Takashima Y, Taniguchi T, Yoshida M, et al. Ocular hypotensive mechanism of intravitreally injected brain natriuretic peptide in rabbit. Investigative ophthalmology & visual science, 1996, 37(13): 2671-2677. | |

BNP (1-32), human Dilution Calculator

BNP (1-32), human Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Basic natriuretic peptide (BNP), now known as B-type natriuretic peptide (also BNP) or GC-B, is a 32 amino acid polypeptide secreted by the ventricles of the heart in response to excessive stretching of heart muscle cells (cardiomyocytes). The release of BNP is modulated by calcium ions(1). BNP is named as such because it was originally identified in extracts of porcine brain, although in humans it is produced mainly in the cardiac ventricles. The physiologic actions of BNP are similar to ANP and include decrease in systemic vascular resistance and central venous pressure as well as an increase in natriuresis. Thus, the net effect of BNP and ANP is a decrease in blood volume which lowers systemic blood pressure and afterload, yielding an increase in cardiac output, partly due to a higher ejection fraction. The main clinical utility of either BNP or NT-BNP is that a normal level rules out acute heart failure in the emergency setting(2). Recombinant BNP, Nesiritide is used to treat decompensated heart failure(3).
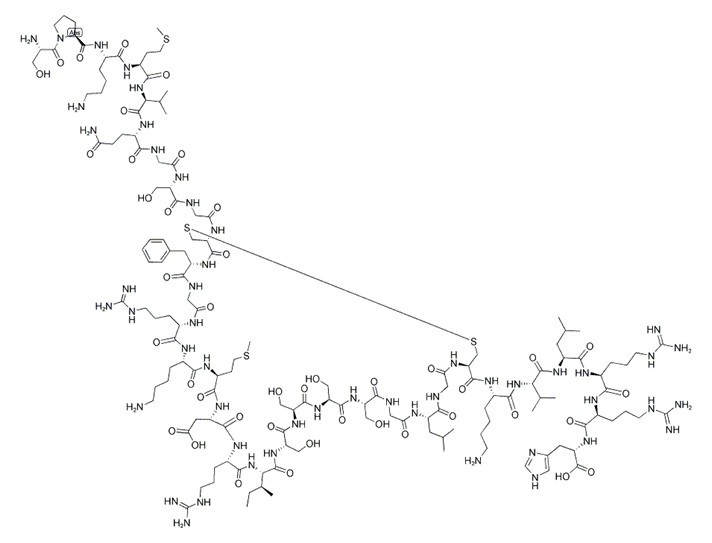
Figure1 Formula of BNP 1-32
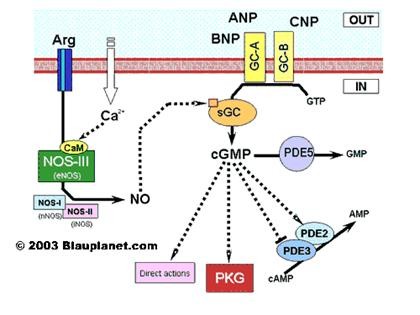
Figure2 signal pathway of Basic natriuretic peptide
Ref:
1. Ziskoven D, Forssmann WG, Holthausen U, Menz G, Addicks K, Rippegater G: Calcium Calmodulinantagonists Influences the release of Cardiodilatin/ANP from Atrial Cardiocytes. Handbook Endocrinology of the Heart, edited by Kaufmann W, Wambach G, 01/1989; Springer Verlag Berlin Heidelberg New York;
2. Maisel A, Krishnaswamy P, Nowak R, McCord J, Hollander J, Duc P, Omland T, Storrow A, Abraham W, Wu A, Clopton P, Steg P, Westheim A, Knudsen C, Perez A, Kazanegra R, Herrmann H, McCullough P (2002). "Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure". N Engl J Med 347 (3): 161–7.
3. O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. TheNew Englandjournal of medicine 2011;365:32-43.
- Beta-Furoyleupatolide
Catalog No.:BCN6407
CAS No.:114437-24-0
- WYE-125132 (WYE-132)
Catalog No.:BCC4608
CAS No.:1144068-46-1
- PF-8380
Catalog No.:BCC1857
CAS No.:1144035-53-9
- Fmoc-D-Leu-OH
Catalog No.:BCC3511
CAS No.:114360-54-2
- TAK 21d
Catalog No.:BCC5609
CAS No.:1143578-94-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- Guanosine Hydrate
Catalog No.:BCC5326
CAS No.:1143525-19-2
- Soyacerebroside I
Catalog No.:BCN6022
CAS No.:114297-20-0
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
- Puerarin 6''-O-xyloside
Catalog No.:BCN2780
CAS No.:114240-18-5
- PAOPA
Catalog No.:BCC6353
CAS No.:114200-31-6
- Z-Ala-OH
Catalog No.:BCC3055
CAS No.:1142-20-7
- Oroxin B
Catalog No.:BCN1203
CAS No.:114482-86-9
- Z-Ser-OH
Catalog No.:BCC2743
CAS No.:1145-80-8
- (-)-U-50488 hydrochloride
Catalog No.:BCC6666
CAS No.:114528-79-9
- (+)-U-50488 hydrochloride
Catalog No.:BCC6656
CAS No.:114528-81-3
- Spiramine A
Catalog No.:BCN6023
CAS No.:114531-28-1
- Honyucitrin
Catalog No.:BCN4728
CAS No.:114542-44-8
- Regelidine
Catalog No.:BCN3094
CAS No.:114542-54-0
- Galanin (1-29) (rat, mouse)
Catalog No.:BCC5928
CAS No.:114547-31-8
- Boeravinone B
Catalog No.:BCN6466
CAS No.:114567-34-9
- Ganoderiol F
Catalog No.:BCN6024
CAS No.:114567-47-4
- Coronadiene
Catalog No.:BCN3683
CAS No.:1145689-64-0
- Thevebioside
Catalog No.:BCN6025
CAS No.:114586-47-9
Specificity of B-Type Natriuretic Peptide Assays: Cross-Reactivity with Different BNP, NT-proBNP, and proBNP Peptides.[Pubmed:28062628]
Clin Chem. 2017 Jan;63(1):351-358.
BACKGROUND: B-type natriuretic peptides (BNPs) are used clinically to diagnose and monitor heart failure and are present in the circulation as multiple proBNP-derived fragments. We investigated the specificity of BNP immunoassays with glycosylated and nonglycosylated BNP, N-terminal proBNP (NT-proBNP), and proBNP peptides to probe the cross-reactivity of each assay. METHODS: Nine B-type natriuretic peptides were studied,including synthetic and recombinant BNP (Shionogi, Scios, Mayo), human and synthetic glycosylated and nonglycosylated NT-proBNP (HyTest, Roche Diagnostics), and human glycosylated and nonglycosylated proBNP (HyTest, Scios). Five BNP [Abbott, Abbott POC, Alere, Beckman Coulter, Siemens (Centaur)], 9 NT-proBNP [Ortho-Clinical Diagnostics, Roche, Response, bioMerieux, Siemens (Dimension, Immulite, Stratus CS), Mitsubishi] and 3 research-use-only proBNP immunoassays [Biosite (Alere), Bio-Rad, Goetze] were evaluated. Specificity was assessed by calculating the recovery between baseline and peptide-spiked human plasma pools at target concentrations of 100 ng/L BNP, 300 ng/L proBNP, or 450 ng/L NT-proBNP. All assays were performed in duplicate. RESULTS: BNP and NT-proBNP assays demonstrated substantial cross-reactivity with proBNP peptides. NT-proBNP assays do not detect glycosylated forms of either NT-proBNP or proBNP. proBNP assays preferentially detect the BNP 1-32 peptide and have minimal cross-reactivity with BNP peptides and glycosylated proBNP. CONCLUSIONS: BNP or NT-proBNP results are not transferable among the current existing immunoassays owing to their differences in cross-reactivity and ability to detect various glycosylated forms of proBNP-derived fragments. Opportunities remain to standardize and harmonize BNP and NT-proBNP assays, as well as to develop specific proBNP assays, to widen their clinical scope of use.
Serum Soluble Urokinase-Type Plasminogen Activator Receptor Is Associated with Low Left Ventricular Ejection Fraction and Elevated Plasma Brain-Type Natriuretic Peptide Level.[Pubmed:28135310]
PLoS One. 2017 Jan 30;12(1):e0170546.
BACKGROUND: Recent studies have suggested that soluble urokinase plasminogen activator receptor (suPAR), a biomarker of subclinical levels of inflammation, is significantly correlated with cardiovascular events. PURPOSE: We investigated the association between suPAR and left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI), and plasma B-type natriuretic peptide (BNP) among cardiac inpatients. METHODS AND RESULTS: In total, 242 patients (mean age 71.3 +/- 9.8 years; 70 women) admitted to the cardiology department were enrolled in the study. suPAR was significantly correlated with LVEF (R = -0.24, P<0.001), LVMI (R = 0.16, P = 0.014) and BNP (R = 0.46, P<0.001). In logistic regression analysis, the highest suPAR tertile (> 3236 pg/mL) was associated with low LVEF (< 50%) and elevated BNP (> 300 pg/mL) with an odds ratio of 3.84 (95% confidence interval [CI], 1.22-12.1) and 5.36 (95% CI, 1.32-21.8), respectively, after adjusting for age, sex, log-transformed estimated glomerular filtration rate (log(eGFR)), C-reactive protein, and diuretic use. The association between suPAR and LVMI was not statistically significant. In multivariate receiver operating characteristic analysis, addition of log(suPAR) to the combination of age, sex, log(eGFR) and CRP incrementally improved the prediction of low LVEF (area under the curve [AUC], 0.827 to 0.852, P = 0.046) and BNP >/= 300 pg/mL (AUC, 0.869 to 0.906; P = 0.029). CONCLUSIONS: suPAR was associated with low LVEF and elevated BNP, but not with left ventricular hypertrophy, independent of CRP, renal function, and diuretic use among cardiac inpatients who were not undergoing chronic hemodialysis.
Searching for a BNP standard: Glycosylated proBNP as a common calibrator enables improved comparability of commercial BNP immunoassays.[Pubmed:27823960]
Clin Biochem. 2017 Mar;50(4-5):181-185.
BACKGROUND: Circulating B-type natriuretic peptide (BNP) is widely accepted as a diagnostic and risk assessment biomarker of cardiac function. Studies suggest that there are significant differences in measured concentrations among different commercial BNP immunoassays. The purpose of our study was to compare BNP-related proteins to determine a form that could be used as a common calibrator to improve the comparability of commercial BNP immunoassay results. METHODS: BNP was measured in 40 EDTA-plasma samples from acute and chronic heart failure patients using five commercial BNP assays: Alere Triage, Siemens Centaur XP, Abbott I-STAT, Beckman Access2 and ET Healthcare Pylon. In parallel with internal calibrators from each manufacturer, six preparations containing BNP 1-32 motif a) synthetic BNP, b) recombinant BNP (E. coli), c) recombinant nonglycosylated proBNP (E. coli), d) recombinant His-tagged (N-terminal) nonglycosylated proBNP (E. coli), e) recombinant glycosylated proBNP (HEK cells), and f) recombinant glycosylated proBNP (CHO cells) were also used as external calibrators for each assay. RESULTS: Using the internal standards provided by manufacturers and for five of six external calibrators, up to 3.6-fold differences (mean 1.9-fold) were observed between BNP immunoassays (mean between-assay CV 24.5-47.2%). A marked reduction of the between-assay variability was achieved, when glycosylated proBNP expressed in HEK cells was used as the common calibrator for all assays (mean between-assay CV 14.8%). CONCLUSIONS: Our data suggest that recombinant glycosylated proBNP could serve as a common calibrator for BNP immunoassays to reduce between-assay variability and achieve better comparability of BNP concentrations of commercial BNP immunoassays.
A candidate liquid chromatography mass spectrometry reference method for the quantification of the cardiac marker 1-32 B-type natriuretic peptide.[Pubmed:28426429]
Clin Chem Lab Med. 2017 Aug 28;55(9):1397-1406.
BACKGROUND: B-type natriuretic peptide (BNP) is a 32 amino acid cardiac hormone routinely measured by immunoassays to diagnose heart failure. While it is reported that immunoassay results can vary up to 45%, no attempt of standardization and/or harmonization through the development of certified reference materials (CRMs) or reference measurement procedures (RMPs) has yet been carried out. METHODS: B-type natriuretic peptide primary calibrator was quantified traceably to the International System of Units (SI) by both amino acid analysis and tryptic digestion. A method for the stabilization of BNP in plasma followed by protein precipitation, solid phase extraction (SPE) and liquid chromatography (LC) mass spectrometry (MS) was then developed and validated for the quantification of BNP at clinically relevant concentrations (15-150 fmol/g). RESULTS: The candidate reference method was applied to the quantification of BNP in a number of samples from the UK NEQAS Cardiac Markers Scheme to demonstrate its applicability to generate reference values and to preliminary evaluate the commutability of a potential CRM. The results from the reference method were consistently lower than the immunoassay results and discrepancy between the immunoassays was observed confirming previous data. CONCLUSIONS: The application of the liquid chromatography-mass spectrometry (LC-MS) method to the UK NEQAS samples and the correlation of the results with the immunoassay results shows the potential of the method to support external quality assessment schemes, to improve understanding of the bias of the assays and to establish RMPs for BNP measurements. Furthermore, the method has the potential to be multiplexed for monitoring circulating truncated forms of BNP.
Validation of a B-type natriuretic peptide as a prognostic marker in pneumonia patients: a prospective cohort study.[Pubmed:26908529]
BMJ Open. 2016 Feb 23;6(2):e010440.
OBJECTIVES: To validate a B-type natriuretic peptide (BNP) as a prognostic marker in pneumonia patients. DESIGN: A prospective cohort study. SETTING: Kanazawa Medical University Himi Municipal (a 250-bed community hospital in Himi-shi, Toyama-ken, Japan). PARTICIPANTS: All patients diagnosed with pneumonia by the physician and admitted to our hospital between 1 January 2012 and 31 March 2015 whose BNP levels had been determined in the first 24 h of admission. A total of 673 patients were enrolled. Of these, BNP levels were measured for a total of 369 patients on admission. INTERVENTION: After enrolment, baseline, demographic, clinical and laboratory characteristics including levels of suspected prognostic markers for pneumonia proposed in previous papers, were collected. All patients were followed up until discharge. During analysis, they were divided into categories as follows: community-acquired pneumonia (CAP), aspiration pneumonia (AP), healthcare-associated pneumonia (HCAP) and pneumonia with acute heart failure (PAHF). A univariate and multivariable Cox-regression analysis were applied to each parameter to identify predictors of death. Three cut-off points, namely 40, 100 and 200 pg/mL, as well as the mean, were applied when comparing BNP levels. MAIN OUTCOME MEASURES: 30-day mortality. RESULTS: Of the 369 patients finally included, 137 were diagnosed with CAP, 122 with AP, 74 with HCAP, and 36 with PAHF. In the univariate analysis, BNP levels (mean, cut-off points 100 pg/mL and 200 pg/mL, p<0.01, respectively) were associated with death in CAP, and similar situation was found for BNP (cut-off points 200 pg/mL, p<0.05) in AP, but not for HCAP, or PAHF. In multivariable Cox-regression analysis, BNP remained an independent mortality predictor (HR 10.01, 95% CI 1.32 to 75.7, p=0.03) in CAP. CONCLUSIONS: BNP levels may be a useful single prognostic marker for CAP. Further research for validation is warranted.


