PAOPADopamine D2 receptor allosteric modulator CAS# 114200-31-6 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114200-31-6 | SDF | Download SDF |
| PubChem ID | 44281634 | Appearance | Powder |
| Formula | C11H18N4O3 | M.Wt | 254.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
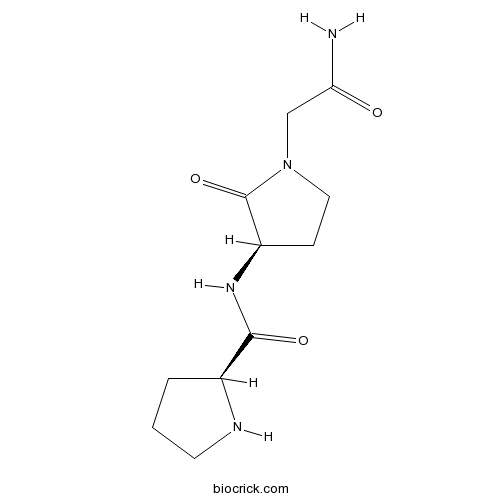
| Chemical Name | (2S)-N-[(3R)-1-(2-amino-2-oxoethyl)-2-oxopyrrolidin-3-yl]pyrrolidine-2-carboxamide | ||
| SMILES | C1CC(NC1)C(=O)NC2CCN(C2=O)CC(=O)N | ||
| Standard InChIKey | QXHVGEXNEZRSGG-JGVFFNPUSA-N | ||
| Standard InChI | InChI=1S/C11H18N4O3/c12-9(16)6-15-5-3-8(11(15)18)14-10(17)7-2-1-4-13-7/h7-8,13H,1-6H2,(H2,12,16)(H,14,17)/t7-,8+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Allosteric modulator of dopamine D2 receptors. Prevents and reverses behavioral and biochemical abnormalities in an amphetamine-sensitized animal model of schizophrenia. |

PAOPA Dilution Calculator

PAOPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9325 mL | 19.6626 mL | 39.3252 mL | 78.6504 mL | 98.3129 mL |
| 5 mM | 0.7865 mL | 3.9325 mL | 7.865 mL | 15.7301 mL | 19.6626 mL |
| 10 mM | 0.3933 mL | 1.9663 mL | 3.9325 mL | 7.865 mL | 9.8313 mL |
| 50 mM | 0.0787 mL | 0.3933 mL | 0.7865 mL | 1.573 mL | 1.9663 mL |
| 100 mM | 0.0393 mL | 0.1966 mL | 0.3933 mL | 0.7865 mL | 0.9831 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Z-Ala-OH
Catalog No.:BCC3055
CAS No.:1142-20-7
- Butyraxanthone B
Catalog No.:BCN3603
CAS No.:1141754-81-5
- Kuguacin N
Catalog No.:BCN3056
CAS No.:1141453-73-7
- Kuguacin J
Catalog No.:BCN3055
CAS No.:1141453-65-7
- Tirandalydigin
Catalog No.:BCN1860
CAS No.:114118-91-1
- Cabozantinib malate (XL184)
Catalog No.:BCC4388
CAS No.:1140909-48-3
- Ibandronic acid
Catalog No.:BCC5204
CAS No.:114084-78-5
- Humantenidine
Catalog No.:BCN4754
CAS No.:114027-39-3
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
- Phaclofen
Catalog No.:BCC6562
CAS No.:114012-12-3
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Puerarin 6''-O-xyloside
Catalog No.:BCN2780
CAS No.:114240-18-5
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
- Soyacerebroside I
Catalog No.:BCN6022
CAS No.:114297-20-0
- Guanosine Hydrate
Catalog No.:BCC5326
CAS No.:1143525-19-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- TAK 21d
Catalog No.:BCC5609
CAS No.:1143578-94-2
- Fmoc-D-Leu-OH
Catalog No.:BCC3511
CAS No.:114360-54-2
- PF-8380
Catalog No.:BCC1857
CAS No.:1144035-53-9
- WYE-125132 (WYE-132)
Catalog No.:BCC4608
CAS No.:1144068-46-1
- Beta-Furoyleupatolide
Catalog No.:BCN6407
CAS No.:114437-24-0
- BNP (1-32), human
Catalog No.:BCC1039
CAS No.:114471-18-0
- Oroxin B
Catalog No.:BCN1203
CAS No.:114482-86-9
Effects of the dopamine D2 allosteric modulator, PAOPA, on the expression of GRK2, arrestin-3, ERK1/2, and on receptor internalization.[Pubmed:23940634]
PLoS One. 2013 Aug 6;8(8):e70736.
The activity of G protein-coupled receptors (GPCRs) is intricately regulated by a range of intracellular proteins, including G protein-coupled kinases (GRKs) and arrestins. Understanding the effects of ligands on these signaling pathways could provide insights into disease pathophysiologies and treatment. The dopamine D2 receptor is a GPCR strongly implicated in the pathophysiology of a range of neurological and neuropsychiatric disorders, particularly schizophrenia. Previous studies from our lab have shown the preclinical efficacy of a novel allosteric drug, 3(R)-[(2(S)-pyrrolidinylcarbonyl)amino]-2-oxo-1-pyrrolidineacetamide (PAOPA), in attenuating schizophrenia-like behavioural abnormalities in rodent models of the disease. As an allosteric modulator, PAOPA binds to a site on the D2 receptor, which is distinct from the endogenous ligand-binding site, in order to modulate the binding of the D2 receptor ligand, dopamine. The exact signaling pathways affected by this allosteric modulator are currently unknown. The objectives of this study were to decipher the in vivo effects, in rats, of chronic PAOPA administration on D2 receptor regulatory and downstream molecules, including GRK2, arrestin-3 and extracellular receptor kinase (ERK) 1/2. Additionally, an in vitro cellular model was also used to study PAOPA's effects on D2 receptor internalization. Results from western immunoblots showed that chronic PAOPA treatment increased the striatal expression of GRK2 by 41%, arrestin-3 by 34%, phospho-ERK1 by 51% and phospho-ERK2 by 36%. Results also showed that the addition of PAOPA to agonist treatment in cells increased D2 receptor internalization by 33%. This study provides the foundational evidence of putative signaling pathways, and changes in receptor localization, affected by treatment with PAOPA. It improves our understanding on the diverse mechanisms of action of allosteric modulators, while advancing PAOPA's development into a novel drug for the improved treatment of schizophrenia.
Preclinical pharmacokinetic and toxicological evaluation of MIF-1 peptidomimetic, PAOPA: examining the pharmacology of a selective dopamine D2 receptor allosteric modulator for the treatment of schizophrenia.[Pubmed:23416534]
Peptides. 2013 Apr;42:89-96.
Schizophrenia is a mental illness characterized by a breakdown in cognition and emotion. Over the years, drug treatment for this disorder has mainly been compromised of orthosteric ligands that antagonize the active site of the dopamine D2 receptor. However, these drugs are limited in their use and often lead to the development of adverse movement and metabolic side effects. Allosteric modulators are an emerging class of therapeutics with significant advantages over orthosteric ligands, including an improved therapeutic and safety profile. This study investigates our newly developed allosteric modulator, PAOPA, which is a specific modulator of the dopamine D2 receptor. Previous studies have shown PAOPA to attenuate schizophrenia-like behavioral abnormalities in preclinical models. To advance this newly developed allosteric drug from the preclinical to clinical stage, this study examines the pharmacokinetic behavior and toxicological profile of PAOPA. Results from this study prove the effectiveness of PAOPA in reaching the implicated regions of the brain for therapeutic action, particularly the striatum. Pharmacokinetic parameters of PAOPA were found to be comparable to current market antipsychotic drugs. Necropsy and histopathological analyses showed no abnormalities in all examined organs. Acute and chronic treatment of PAOPA indicated no movement abnormalities commonly found with the use of current typical antipsychotic drugs. Moreover, acute and chronic PAOPA treatment revealed no hematological or metabolic abnormalities classically found with the use of atypical antipsychotic drugs. Findings from this study demonstrate a better safety profile of PAOPA, and necessitates the progression of this newly developed therapeutic for the treatment of schizophrenia.
PAOPA, a potent dopamine D2 receptor allosteric modulator, prevents and reverses behavioral and biochemical abnormalities in an amphetamine-sensitized preclinical animal model of schizophrenia.[Pubmed:22658400]
Eur Neuropsychopharmacol. 2013 Mar;23(3):253-62.
Allosteric modulators are emerging as new therapeutics for the treatment of psychiatric illnesses, such as schizophrenia. Conventional antipsychotic drugs are typically dopamine D2 receptor antagonists that compete with endogenous dopamine at the orthosteric site, and block excessive dopamine neurotransmission in the brain. However, they are unable to treat all symptoms of schizophrenia and often cause adverse motor and metabolic side effects. The binding profile of allosteric modulators differs, as they interact with their receptor at a novel binding site and their activity is determined by physiological signaling. In collaboration, our laboratories have synthesized and evaluated over 185 compounds for their allosteric modulatory activity at the dopamine D2 receptor. Of these compounds, PAOPA is among the most potent allosteric modulators, and has been shown to be effective in treating the MK-801 induced preclinical animal model of schizophrenia. The objective of this study was to evaluate PAOPA's ability to prevent and reverse behavioral abnormalities in an amphetamine-sensitized preclinical animal model of schizophrenia. Amphetamine sensitized rats were given PAOPA during sensitization and following sensitization to determine whether PAOPA is able to prevent and reverse behavioral abnormalities. Furthermore, changes in post-mortem dopamine levels were measured by high performance liquid chromatography in various brain regions. The results presented demonstrate that PAOPA is able to prevent and reverse behavioral and biochemical abnormalities in an amphetamine-sensitized animal model of schizophrenia.
Change in expression of vesicular protein synapsin II by chronic treatment with D2 allosteric modulator PAOPA.[Pubmed:25703303]
Peptides. 2015 Apr;66:58-62.
The hallmark symptoms of schizophrenia include profound disturbances in thought, perception, cognition etc., which negatively impacts an individual's quality of life. Current antipsychotic drugs are not effective in treating all symptoms of this disorder, and often cause severe movement and metabolic side effects. Consequently, there remains a strong impetus to develop safer and more efficacious therapeutics for patients, as well as elucidating the etiology of schizophrenia. Previous work in our lab has introduced a novel candidate for the treatment of this disease: the dopamine D2 receptor (D2R) allosteric modulator, 3(R)-[(2(S)-pyrrolidinylcarbonyl)amino]-2-oxo-1-pyrrolidineacetamide (PAOPA). We have previously shown that PAOPA, by selectively modulating D2R, can ameliorate schizophrenia-like symptoms in animal models, although the precise mechanism is presently not understood. Synapsin II is a presynaptic vesicular protein which has been strongly implicated in schizophrenia, as it is reduced in the prefrontal cortex of patients, and knockdown of this protein elicits schizophrenia-like phenotypes in animal models. Given the therapeutic effects of PAOPA and the role of synapsin II in schizophrenia, the objective of this study was to investigate the effect of chronic administration of PAOPA (45 days) on neuronal synapsin II protein expression in rodents. Immunoblot results revealed that the synapsin IIa, but not the IIb isoform, was increased in the dopaminergic regions of the striatum, nucleus accumbens, and medial prefrontal cortex. The results of this study implicate a role for modulation of synapsin II as a possible therapeutic mechanism of action for potential antipsychotic drug PAOPA.
PAOPA, a potent analogue of Pro-Leu-glycinamide and allosteric modulator of the dopamine D2 receptor, prevents NMDA receptor antagonist (MK-801)-induced deficits in social interaction in the rat: implications for the treatment of negative symptoms in schizophrenia.[Pubmed:21036015]
Schizophr Res. 2011 Jan;125(1):88-92.
The aim of this study was to investigate whether a potent analogue of the endogenous brain peptide l-prolyl-l-leucyl-glycinamide (PLG), (3(R)-[(2(S)-pyrrolidinylcarbonyl)amino]-2-oxo-1-pyrrolidineacetamide (PAOPA), can prevent the induction of social withdrawal caused by sub-chronic treatment with the non-competitive NMDA (N-methyl-l-aspartate) receptor antagonist, MK-801. Results indicate that MK-801 (0.5 mg/kg) significantly decreased social interaction following sub-chronic treatment (7 days). Treatment with PAOPA (1 mg/kg) blocked the effects of MK-801, and increased the amount of time spent in social interaction in comparison to control animals. These results provide evidence for the development of peptidomimetic compounds for the treatment of social withdrawal and related negative symptoms associated with schizophrenia.


