HumantenidineCAS# 114027-39-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114027-39-3 | SDF | Download SDF |
| PubChem ID | 14217347 | Appearance | Powder |
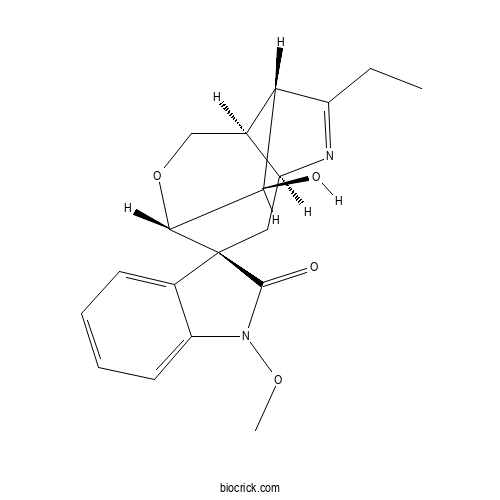
| Formula | C19H22N2O4 | M.Wt | 342.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 14-hydroxygelsenicine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,2S,4S,7R,8S,11R)-6-ethyl-11-hydroxy-1'-methoxyspiro[10-oxa-5-azatricyclo[5.3.1.04,8]undec-5-ene-2,3'-indole]-2'-one | ||
| SMILES | CCC1=NC2CC3(C4C(C1C2CO4)O)C5=CC=CC=C5N(C3=O)OC | ||
| Standard InChIKey | FLDAHLDTMBMPJD-HCWKNHCHSA-N | ||
| Standard InChI | InChI=1S/C19H22N2O4/c1-3-12-15-10-9-25-17(16(15)22)19(8-13(10)20-12)11-6-4-5-7-14(11)21(24-2)18(19)23/h4-7,10,13,15-17,22H,3,8-9H2,1-2H3/t10-,13+,15-,16-,17-,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | Cytotoxic steroids of Gelsemium sempervirens.[Pubmed: 3655795]J Nat Prod. 1987 Mar-Apr;50(2):195-8.
|
| Structure Identification | J Sep Sci. 2014 Feb;37(3):308-13.Ultrasound/microwave-assisted extraction and comparative analysis of bioactive/toxic indole alkaloids in different medicinal parts of Gelsemium elegans Benth by ultra-high performance liquid chromatography with MS/MS.[Pubmed: 24375943 ]Indole alkaloids are the main bioactive/toxic components in Gelsemium elegans Benth.
|

Humantenidine Dilution Calculator

Humantenidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9206 mL | 14.6028 mL | 29.2056 mL | 58.4112 mL | 73.014 mL |
| 5 mM | 0.5841 mL | 2.9206 mL | 5.8411 mL | 11.6822 mL | 14.6028 mL |
| 10 mM | 0.2921 mL | 1.4603 mL | 2.9206 mL | 5.8411 mL | 7.3014 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5841 mL | 1.1682 mL | 1.4603 mL |
| 100 mM | 0.0292 mL | 0.146 mL | 0.2921 mL | 0.5841 mL | 0.7301 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
- Phaclofen
Catalog No.:BCC6562
CAS No.:114012-12-3
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Scopolamine hydrobromide
Catalog No.:BCN1199
CAS No.:114-49-8
- Erythromycin
Catalog No.:BCC4778
CAS No.:114-07-8
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- Ibandronic acid
Catalog No.:BCC5204
CAS No.:114084-78-5
- Cabozantinib malate (XL184)
Catalog No.:BCC4388
CAS No.:1140909-48-3
- Tirandalydigin
Catalog No.:BCN1860
CAS No.:114118-91-1
- Kuguacin J
Catalog No.:BCN3055
CAS No.:1141453-65-7
- Kuguacin N
Catalog No.:BCN3056
CAS No.:1141453-73-7
- Butyraxanthone B
Catalog No.:BCN3603
CAS No.:1141754-81-5
- Z-Ala-OH
Catalog No.:BCC3055
CAS No.:1142-20-7
- PAOPA
Catalog No.:BCC6353
CAS No.:114200-31-6
- Puerarin 6''-O-xyloside
Catalog No.:BCN2780
CAS No.:114240-18-5
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
- Soyacerebroside I
Catalog No.:BCN6022
CAS No.:114297-20-0
- Guanosine Hydrate
Catalog No.:BCC5326
CAS No.:1143525-19-2
Cytotoxic steroids of Gelsemium sempervirens.[Pubmed:3655795]
J Nat Prod. 1987 Mar-Apr;50(2):195-8.
A new pregnane derivative, 12 beta-hydroxy-5 alpha-pregn-16-ene-3,20-dione, along with the known derivative 12 beta-hydroxy-pregna-4,16-diene-3,20-dione have been isolated from a MeOH extract of the stem of Gelsemium sempervirens and found to be the principal cytotoxic entities. The 13C-nmr spectra of both compounds were assigned by comparison with other pregnane analogs thereby allowing confirmation of the stereochemistry at C-5 in compound. Heteronuclear 2D correlation and selective INEPT experiments indicated the need to revise a number of 13C-nmr assignments of pregn-4,16-dien-3,20-dione. Nine indole alkaloids, gelsemine, gelsevirine, 21-oxogelsemine, gelsedine, 14 beta-hydroxygelsedine, gelsenicine, Humantenidine, humantenirine, and koumidine were found to be inactive in the KB and P-388 cytotoxicity test systems.
Ultrasound/microwave-assisted extraction and comparative analysis of bioactive/toxic indole alkaloids in different medicinal parts of Gelsemium elegans Benth by ultra-high performance liquid chromatography with MS/MS.[Pubmed:24375943]
J Sep Sci. 2014 Feb;37(3):308-13.
Indole alkaloids are the main bioactive/toxic components in Gelsemium elegans Benth. To determine the distribution and contents of indole alkaloids in its different medicinal parts, a novel and rapid method using ultra-high performance LC (UPLC) with MS/MS has been established and validated with an optimized ultrasound/microwave-assisted extraction method. Four constituents, namely, Humantenidine, humantenmine, gelsemine, and koumine, were simultaneously determined in 6 min. Chromatographic separation was achieved on an ultra-high performance LC BEH C18 column with a gradient mobile phase consisting of methanol and water (containing 0.1% formic acid both in methanol and water) at a flow rate of 0.3 mL/min. The detection was performed on a triple quadrupole electrospray MS/MS by positive ion multiple-reaction monitoring mode. All the analytes showed good linearity (r >/= 0.9934) within a concentration range from 0.1-25 mug/mL with a LOQ of 25-50 ng/mL. The overall intra- and intervariations of four components were <4.7% with an accuracy of 97.3-101.3%. The analysis results showed that there were remarkable differences in the distribution and contents of four chemical markers in the roots, stems, and leaves of G. elegans Benth. The findings can provide necessary and meaningful information for the rational utilization of its resources.


