Kuguacin JCAS# 1141453-65-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1141453-65-7 | SDF | Download SDF |
| PubChem ID | 25243357 | Appearance | Powder |
| Formula | C30H46O3 | M.Wt | 454.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
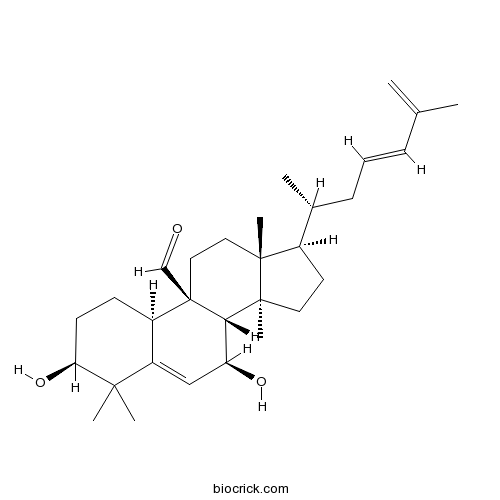
| Chemical Name | (3S,7S,8S,9R,10R,13R,14S,17R)-3,7-dihydroxy-4,4,13,14-tetramethyl-17-[(2R,4E)-6-methylhepta-4,6-dien-2-yl]-2,3,7,8,10,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthrene-9-carbaldehyde | ||
| SMILES | CC(CC=CC(=C)C)C1CCC2(C1(CCC3(C2C(C=C4C3CCC(C4(C)C)O)O)C=O)C)C | ||
| Standard InChIKey | JWZXELQQTJCVII-KPBOVSLYSA-N | ||
| Standard InChI | InChI=1S/C30H46O3/c1-19(2)9-8-10-20(3)21-13-14-29(7)26-24(32)17-23-22(11-12-25(33)27(23,4)5)30(26,18-31)16-15-28(21,29)6/h8-9,17-18,20-22,24-26,32-33H,1,10-16H2,2-7H3/b9-8+/t20-,21-,22-,24+,25+,26+,28-,29+,30-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kuguacin J exerts growth inhibition through G1 arrest and induction of apoptosis in androgen-dependent human prostate cancer, it is a promising candidate new antineoplastic and chemopreventive agent for androgen-dependent prostate cancer and carcinogenesis. 2. Kuguacin J modulates the function of P-glycoprotein (P-gp) by directly interacting at the drug-substrate-binding site, and it appears to be an effective inhibitor of P-gp activity in vitro and thus could be developed as an effective chemosensitizer to treat multidrug-resistant cancers. |
| Targets | p21 | CDK | Bcl-2/Bax | Caspase | PARP | Androgen Receptor | p53 | P-gp |

Kuguacin J Dilution Calculator

Kuguacin J Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1993 mL | 10.9963 mL | 21.9925 mL | 43.985 mL | 54.9813 mL |
| 5 mM | 0.4399 mL | 2.1993 mL | 4.3985 mL | 8.797 mL | 10.9963 mL |
| 10 mM | 0.2199 mL | 1.0996 mL | 2.1993 mL | 4.3985 mL | 5.4981 mL |
| 50 mM | 0.044 mL | 0.2199 mL | 0.4399 mL | 0.8797 mL | 1.0996 mL |
| 100 mM | 0.022 mL | 0.11 mL | 0.2199 mL | 0.4399 mL | 0.5498 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tirandalydigin
Catalog No.:BCN1860
CAS No.:114118-91-1
- Cabozantinib malate (XL184)
Catalog No.:BCC4388
CAS No.:1140909-48-3
- Ibandronic acid
Catalog No.:BCC5204
CAS No.:114084-78-5
- Humantenidine
Catalog No.:BCN4754
CAS No.:114027-39-3
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
- Phaclofen
Catalog No.:BCC6562
CAS No.:114012-12-3
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Scopolamine hydrobromide
Catalog No.:BCN1199
CAS No.:114-49-8
- Erythromycin
Catalog No.:BCC4778
CAS No.:114-07-8
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Kuguacin N
Catalog No.:BCN3056
CAS No.:1141453-73-7
- Butyraxanthone B
Catalog No.:BCN3603
CAS No.:1141754-81-5
- Z-Ala-OH
Catalog No.:BCC3055
CAS No.:1142-20-7
- PAOPA
Catalog No.:BCC6353
CAS No.:114200-31-6
- Puerarin 6''-O-xyloside
Catalog No.:BCN2780
CAS No.:114240-18-5
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
- Soyacerebroside I
Catalog No.:BCN6022
CAS No.:114297-20-0
- Guanosine Hydrate
Catalog No.:BCC5326
CAS No.:1143525-19-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- TAK 21d
Catalog No.:BCC5609
CAS No.:1143578-94-2
- Fmoc-D-Leu-OH
Catalog No.:BCC3511
CAS No.:114360-54-2
- PF-8380
Catalog No.:BCC1857
CAS No.:1144035-53-9
Kuguacin J isolated from Momordica charantia leaves inhibits P-glycoprotein (ABCB1)-mediated multidrug resistance.[Pubmed:21414769]
J Nutr Biochem. 2012 Jan;23(1):76-84.
Multidrug resistance (MDR) is a major factor in the failure of chemotherapy in cancer patients. Resistance to chemotherapy has been correlated to the overexpression of ABC drug transporters including P-glycoprotein (P-gp) that actively efflux chemotherapeutic drugs from cancer cells. Our previous study showed that bitter melon (Momordica charantia) leaf extract (BMLE) was able to reverse the MDR phenotype by increasing the intracellular accumulation of chemotherapeutic drugs. In the present study, bioguided fractionation was used to identify the active component(s) of BMLE that is able to modulate the function of P-gp and the MDR phenotype in a human cervical carcinoma cell line (KB-V1). We found that Kuguacin J, one of the active components in BMLE, increased sensitivity to vinblastine and paclitaxel in KB-V1 cells. A flow cytometry assay indicated that Kuguacin J inhibits the transport function of P-gp and thereby significantly increases the accumulation of rhodamine 123 and calcein AM in the cells. These results were confirmed by [(3)H]-vinblastine transport assay. Kuguacin J significantly increases intracellular [(3)H]-vinblastine accumulation and decreased the [(3)H]-vinblastine efflux in the cells. Kuguacin J also inhibited the incorporation of [(1)(2)(5)I]-iodoarylazidoprazosin into P-gp in a concentration-dependent manner, indicating that Kuguacin J directly interacts with the drug-substrate-binding site on P-gp. These results indicate that Kuguacin J modulates the function of P-gp by directly interacting at the drug-substrate-binding site, and it appears to be an effective inhibitor of P-gp activity in vitro and thus could be developed as an effective chemosensitizer to treat multidrug-resistant cancers.
Induction of G1 arrest and apoptosis in androgen-dependent human prostate cancer by Kuguacin J, a triterpenoid from Momordica charantia leaf.[Pubmed:21429659]
Cancer Lett. 2011 Jul 28;306(2):142-50.
In this study, we focused on the effects of a bitter melon (Momordica charantia) leaf extract (BMLE) and a purified component, Kuguacin J (KuJ), on androgen-dependent LNCaP human prostate cancer cells. Both treatments exerted growth inhibition through G1 arrest and induction of apoptosis. In addition, KuJ markedly decreased the levels of cyclins (D1 and E), cyclin-dependent kinases (Cdk2 and Cdk4) and proliferating cell nuclear antigen, and caused an increase in p21 and p27 levels. Its induction of apoptosis was accompanied by an increase in cleavage of caspase-3 and poly (ADP-ribose) polymerase, attributable to augment of Bax/Bcl-2 and Bad/Bcl-xL and reduction of survivin levels. BMLE and KuJ also reduced the expression of androgen receptor (AR), prostate-specific antigen (PSA) while induced P53 protein level. Down-regulation of p53 by RNA interference indicated that BMLE and KuJ inhibited cell growth partly through p53-dependent cell cycle arrest and apoptotic pathways. Both BMLE and KuJ caused less toxicity in a normal prostate cell line, PNT1A. Our results suggest that BMLE and a purified component, KuJ, from its diethyl ether fraction could be promising candidate new antineoplastic and chemopreventive agents for androgen-dependent prostate cancer and carcinogenesis.


