ErythromycinCAS# 114-07-8 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- XCT790
Catalog No.:BCC5121
CAS No.:725247-18-7
- Toremifene Citrate
Catalog No.:BCC4487
CAS No.:89778-27-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114-07-8 | SDF | Download SDF |
| PubChem ID | 12560 | Appearance | Powder |
| Formula | C37H67NO13 | M.Wt | 733.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
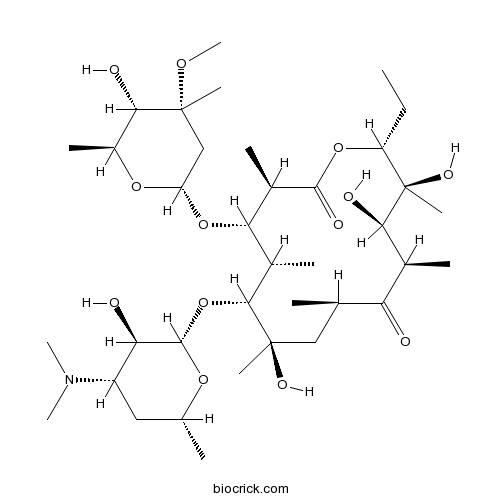
| Chemical Name | (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione | ||
| SMILES | CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O | ||
| Standard InChIKey | ULGZDMOVFRHVEP-RWJQBGPGSA-N | ||
| Standard InChI | InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Erythromycin, an oral macrolide antibiotic produced by Streptomyces erythreus, reversibly binds to the 50S ribosome of bacteria, and inhibits protein synthesis.
Target: Antibacterial
Erythromycin is a macrolide antibiotic that has an antimicrobial spectrum similar to or slightly wider than that of penicillin, and is often prescribed for people who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including Mycoplasma and legionellosis. It was first marketed by Eli Lilly and Company, and it is today commonly known as EES (erythromycin ethylsuccinate, an ester prodrug that is commonly administered). It is also occasionally used as a prokinetic agent.
Erythromycin estolate has been associated with reversible hepatotoxicity in pregnant women in the form of elevated serum glutamic-oxaloacetic transaminase and is not recommended during pregnancy. Some evidence suggests similar hepatotoxicity in other populations. Erythromycin displays bacteriostatic activity or inhibits growth of bacteria, especially at higher concentrations, but the mechanism is not fully understood. By binding to the 50s subunit of the bacterial 70s rRNA complex, protein synthesis and subsequent structure and function processes critical for life or replication are inhibited. Erythromycin interferes with aminoacyl translocation, preventing the transfer of the tRNA bound at the A site of the rRNA complex to the P site of the rRNA complex. Without this translocation, the A site remains occupied and, thus, the addition of an incoming tRNA and its attached amino acid to the nascent polypeptide chain is inhibited. This interferes with the production of functionally useful proteins, which is the basis of this antimicrobial action. References: | |||||

Erythromycin Dilution Calculator

Erythromycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3625 mL | 6.8126 mL | 13.6253 mL | 27.2506 mL | 34.0632 mL |
| 5 mM | 0.2725 mL | 1.3625 mL | 2.7251 mL | 5.4501 mL | 6.8126 mL |
| 10 mM | 0.1363 mL | 0.6813 mL | 1.3625 mL | 2.7251 mL | 3.4063 mL |
| 50 mM | 0.0273 mL | 0.1363 mL | 0.2725 mL | 0.545 mL | 0.6813 mL |
| 100 mM | 0.0136 mL | 0.0681 mL | 0.1363 mL | 0.2725 mL | 0.3406 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Erythromycin, an oral macrolide antibiotic produced by Streptomyces erythreus, reversibly binds to the 50S ribosome of bacteria, and inhibits protein synthesis.
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Scopolamine hydrobromide
Catalog No.:BCN1199
CAS No.:114-49-8
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Phaclofen
Catalog No.:BCC6562
CAS No.:114012-12-3
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
- Humantenidine
Catalog No.:BCN4754
CAS No.:114027-39-3
- Ibandronic acid
Catalog No.:BCC5204
CAS No.:114084-78-5
- Cabozantinib malate (XL184)
Catalog No.:BCC4388
CAS No.:1140909-48-3
- Tirandalydigin
Catalog No.:BCN1860
CAS No.:114118-91-1
- Kuguacin J
Catalog No.:BCN3055
CAS No.:1141453-65-7
- Kuguacin N
Catalog No.:BCN3056
CAS No.:1141453-73-7
- Butyraxanthone B
Catalog No.:BCN3603
CAS No.:1141754-81-5
Erythromycin infusion prior to endoscopy for acute nonvariceal upper gastrointestinal bleeding: a pilot randomized controlled trial.[Pubmed:28352063]
Korean J Intern Med. 2017 Nov;32(6):1002-1009.
BACKGROUND/AIMS: The aim of this study was to compare the effects of Erythromycin infusion and gastric lavage in order to improve the quality of visualization during emergency upper endoscopy. METHODS: We performed a prospective randomized pilot study. Patients presented with hematemesis or melena within 12 hours and were randomly assigned to the Erythromycin group (intravenous infusion of Erythromycin), gastric lavage group (nasogastric tube placement with gastric lavage), or Erythromycin + gastric lavage group (both Erythromycin infusion and gastric lavage). The primary outcome was satisfactory visualization. Secondary outcomes included identification of a bleeding source, the success rate of hemostasis, duration of endoscopy, complications related to Erythromycin infusion or gastric lavage, number of transfused blood units, rebleeding rate, and bleeding-related mortality. RESULTS: A total of 43 patients were randomly assigned: 14 patients in the Erythromycin group; 15 patients in the gastric lavage group; and 14 patients in the Erythromycin + gastric lavage group. Overall satisfactory visualization was achieved in 81% of patients: 92.8% in the Erythromycin group; 60.0% in the gastric lavage group; and 92.9% in the Erythromycin + gastric lavage group, respectively (p = 0.055). The identification of a bleeding source was possible in all cases. The success rate of hemostasis, duration of endoscopy, and number of transfused blood units did not significantly differ between groups. There were no complications. Rebleeding occurred in three patients (7.0%). Bleeding-related mortality was not reported. CONCLUSIONS: Intravenous Erythromycin infusion prior to emergency endoscopy for acute nonvariceal upper gastrointestinal bleeding seems to provide satisfactory endoscopic visualization.
Erythromycin attenuates metalloprotease/anti-metalloprotease imbalance in cigarette smoke-induced emphysema in rats via the mitogen-activated protein kinase/nuclear factor-kappaB activation pathway.[Pubmed:28358431]
Mol Med Rep. 2017 May;15(5):2983-2990.
The present study investigated whether Erythromycin (ERY) reduces cigarette smoke (CS)-induced emphysema in rats and aimed to determine the anti-inflammatory effect of ERY, which may identify potential treatments for chronic obstructive pulmonary disease. Furthermore, the current study focused on the potential effects on the imbalance between matrix metalloprotease (MMP) and anti-MMP activity, the phosphorylation of mitogen-activated protein kinases (MAPKs) and the nuclear factorkappaB (NFkappaB) signaling pathway. Wistar rats were divided into the following three groups (n=12 each): control (ERY vehicle only, without any CS exposure), CS (animals were exposed to CS for 12 weeks) and CS + ERY (animals were exposed to CS for 12 weeks and received 100 mg/kg/day ERY). The recruitment of inflammatory cells into the bronchoalveolar lavage fluid (BALF) and the histopathology of lung tissue from all groups was evaluated to grade the severity of the emphysema. The expression of MMP2, MMP9 and tissue inhibitor of metalloproteinase1 was evaluated by immunohistochemistry and western blotting. The activation of MAPKs, NFkappaB and inhibitor of NFkappaB (IkappaBalpha), in lung tissues was examined by western blotting. Treatment with ERY resulted in fewer inflammatory cells and cytokines in the BALF, and fewer emphysemaassociated changes in the lungs compared with control. The stimulus of CS promoted the phosphorylation of extracellular signalregulated kinase (ERK)1/2 and p38, but not cJun NH2terminal kinase, thereby inducing the activation of the ERK/MAPK signaling pathway in rats. Furthermore, CS exposure increased the expression of NF-kappaB and decreased the expression of IkappaBalpha. The levels of phosphorylated ERK1/2 and p38 were significantly reduced in rats with CSinduced emphysema when treated with ERY compared with the CS group. The results of the present study therefore indicate that oral administration of ERY may suppress CSinduced emphysema by regulating inflammatory cytokines and the MMP/anti-MMP imbalance via the MAPK/NF-kappaB pathway.
Erythromycin: prophylaxis against recurrent small bowel obstruction.[Pubmed:28356304]
BMJ Support Palliat Care. 2017 Sep;7(3):261-263.
We describe three cases where Erythromycin suspension has been used successfully in preventing recurrence of small bowel obstruction in patients with terminal illness and for whom it proved more effective than standard preparations such as metoclopramide and domperidone. These patients also experienced a longer term benefit over some months. With recent alerts over longer term use of metoclopramide and domperidone, we demonstrate that Erythromycin is a viable alternative prokinetic in patients with terminal illness at risk of small bowel obstruction instead of or alongside metoclopramide and domperidone. More research is required to establish the point at which Erythromycin should be considered in the management of symptoms. In addition, research into the possibility of a viable alternative to Erythromycin is needed.
Functional characterization of a common CYP4F11 genetic variant and identification of functionally defective CYP4F11 variants in erythromycin metabolism and 20-HETE synthesis.[Pubmed:28347661]
Arch Biochem Biophys. 2017 Apr 15;620:43-51.
CYP4F11, together with CYP4F2, plays an important role in the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE) from arachidonic acid. We identified 21 variants by whole exome sequencing, including 4 non-synonymous variants in Korean subjects. The proteins of the wild-type CYP4F11 and the four coding variants (C276R, D315N, D374Y, and D446N) were expressed in Escherichia coli DH5alpha cells and purified to give cytochrome P450-specific carbon monoxide difference spectra. Wild-type CYP4F2 was also expressed and purified to compare its activity with the CYP4F11 wild-type. Wild-type CYP4F11 exhibited the highest maximal clearance for Erythromycin N-demethylase activity followed by the variants D374Y, D446N, C276R, and D315N. In particular, the CYP4F11 D315N protein showed about 50% decrease in intrinsic clearance compared to the wild type. The ability of wild-type CYP4F11 and the variants to synthesize 20-HETE from arachidonic acid was similar; the CYP4F11 D315N variant, however, showed only 68% of wild-type activity. Furthermore, the ability of CYP4F2 to synthesize 20-HETE was 1.7-fold greater than that of CYP4F11. Overall, our results suggest that the metabolism of CYP4F11 substrates may be reduced in individuals carrying the CYP4F11 D315N genetic variant and individuals carrying the common D446N CYP4F11 variant likely exhibit comparable 20-HETE synthesis as individuals expressing wild-type CYP4F11.


