XCT790ERRα agonist CAS# 725247-18-7 |
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 725247-18-7 | SDF | Download SDF |
| PubChem ID | 6918788 | Appearance | Powder |
| Formula | C23H13F9N4O3S | M.Wt | 596.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (27.95 mM; Need ultrasonic) | ||
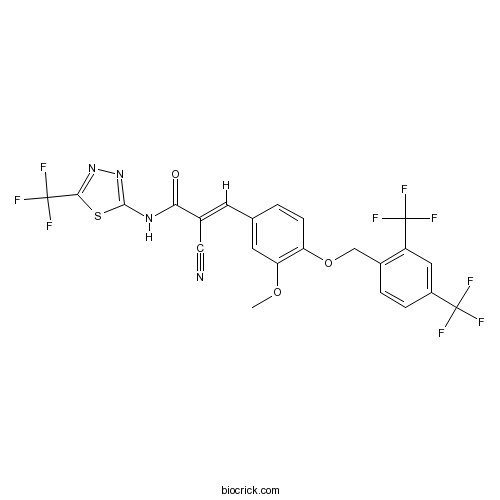
| Chemical Name | (E)-3-[4-[[2,4-bis(trifluoromethyl)phenyl]methoxy]-3-methoxyphenyl]-2-cyano-N-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]prop-2-enamide | ||
| SMILES | COC1=C(C=CC(=C1)C=C(C#N)C(=O)NC2=NN=C(S2)C(F)(F)F)OCC3=C(C=C(C=C3)C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | HQFNFOOGGLSBBT-AWNIVKPZSA-N | ||
| Standard InChI | InChI=1S/C23H13F9N4O3S/c1-38-17-7-11(6-13(9-33)18(37)34-20-36-35-19(40-20)23(30,31)32)2-5-16(17)39-10-12-3-4-14(21(24,25)26)8-15(12)22(27,28)29/h2-8H,10H2,1H3,(H,34,36,37)/b13-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective ERRα antagonist/inverse agonist (IC50 = ~400 nM). Displays no antagonist activity at ERRγ or ERα at concentrations below 10 μM. Interferes with PPARγ coactivator-1α (PGC-1α)/ERRα-dependent signaling: inhibits PGC-1α induction of ERRα and MAO-B gene expression and downregulates the constitutive transcriptional activity of ERRα in numerous cell lines. |

XCT790 Dilution Calculator

XCT790 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6767 mL | 8.3834 mL | 16.7667 mL | 33.5334 mL | 41.9168 mL |
| 5 mM | 0.3353 mL | 1.6767 mL | 3.3533 mL | 6.7067 mL | 8.3834 mL |
| 10 mM | 0.1677 mL | 0.8383 mL | 1.6767 mL | 3.3533 mL | 4.1917 mL |
| 50 mM | 0.0335 mL | 0.1677 mL | 0.3353 mL | 0.6707 mL | 0.8383 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1677 mL | 0.3353 mL | 0.4192 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
XCT790 is an inverse agonist of Estrogen-related receptor alpha (ERRα) with IC50 value of 0.37μM [1].
XCT790 is a first and selective inverse agonist of ERRα. It does not significantly inhibit ERRγ, ERα and ERβ below 10μM. XCT790 is proved to bind ERRα within LBD [1].
As an agonist of ERRα, XCT790 is found to decrease mitochondrial masses as well as affect the mitochondrial membrane potential, results in a subsequent function dysregulation of mitochondrial. XCT790 also reduces the expression level of PGC-1α which is a coactivator of ERRα to regulate mitochondrial biogenesis. Additionally, XCT790 can induce cell death in human hepatocarcinoma cell lines. It reduces the viabilities of HepG2 and its MDR cell line R-HepG2, MES-SA/DX5 and MES-SA in a dose-dependent manner. The mechanism of causing cell death is that XCT790 induces apoptosis through inducing ROS and subsequent caspases activation [2].
References:
[1] Lu X, Peng L, Lv M, ding K. Recent advance in the design of small molecular modulators of estrogen-related receptors. Curr Pharm Des. 2012;18(23):3421-31.
[2] Wu F, Wang J, Wang Y, Kwok TT, Kong SK, Wong C. Estrogen-related receptor alpha (ERRalpha) inverse agonist XCT-790 induces cell death in chemotherapeutic resistant cancer cells. Chem Biol Interact. 2009 Oct 7;181(2):236-42.
- Paeonolide
Catalog No.:BCN2805
CAS No.:72520-92-4
- Specioside
Catalog No.:BCN4280
CAS No.:72514-90-0
- Lycobetaine
Catalog No.:BCN8313
CAS No.:72510-04-4
- Felodipine
Catalog No.:BCC4402
CAS No.:72509-76-3
- Methyl vanillate glucoside
Catalog No.:BCN4033
CAS No.:72500-11-9
- Pirarubicin
Catalog No.:BCC5092
CAS No.:72496-41-4
- STF 31
Catalog No.:BCC7938
CAS No.:724741-75-7
- Psora 4
Catalog No.:BCC7927
CAS No.:724709-68-6
- Praeruptorin C
Catalog No.:BCN4991
CAS No.:72463-77-5
- 11-Hydroxytephrosin
Catalog No.:BCN4861
CAS No.:72458-85-6
- Aniracetam
Catalog No.:BCC4219
CAS No.:72432-10-1
- Miglitol
Catalog No.:BCC4921
CAS No.:72432-03-2
- Polygonal
Catalog No.:BCN4281
CAS No.:72537-20-3
- 6-chloro-9h-fluoren-2-amine
Catalog No.:BCC9231
CAS No.:7254-05-9
- GIP (1-39)
Catalog No.:BCC5890
CAS No.:725474-97-5
- Ceftazidime
Catalog No.:BCC5274
CAS No.:72558-82-8
- Rifabutin
Catalog No.:BCC4936
CAS No.:72559-06-9
- Angeloylgomisin Q
Catalog No.:BCN7033
CAS No.:72561-28-5
- Calebassine
Catalog No.:BCN2276
CAS No.:7257-29-6
- Isosilybin
Catalog No.:BCN2406
CAS No.:72581-71-6
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- γ1-MSH
Catalog No.:BCC6021
CAS No.:72629-65-3
- Prosapogenin CP6
Catalog No.:BCN2535
CAS No.:72629-76-6
Potentiation of ICI182,780 (Fulvestrant)-induced estrogen receptor-alpha degradation by the estrogen receptor-related receptor-alpha inverse agonist XCT790.[Pubmed:17631492]
J Biol Chem. 2007 Sep 28;282(39):28328-34.
ICI182,780 (Fulvestrant) is a pure anti-estrogen used in adjuvant therapies of breast cancer. This compound not only inhibits the transcriptional activities of the estrogen receptor-alpha (ER alpha) but also induces its proteasome-dependent degradation. The latter activity is believed to be required for the antiproliferative effects of ICI182,780. Estrogen receptor-related receptor-alpha (ERR alpha) is an orphan member of the nuclear receptor superfamily that is expressed in a wide range of tissues including breast tumors, in which its high expression correlates with poor prognosis. Although not regulated by any natural ligand, ERR alpha can be deactivated by the synthetic molecule XCT790. Here we demonstrate that this compound also induces a proteasome degradation of ERR alpha. We also show that although it does not act directly on the steady-state level of ER alpha, XCT790 potentiates the ICI182,780-induced ER alpha degradation. We suggest that treatment with XCT790 could thus enhance the efficacy of ICI182,780 in ER alpha-dependent pathologies such as breast cancer.
[XCT790 inhibits rat vascular smooth muscle cells proliferation through down-regulating the expression of estrogen-related receptor alpha].[Pubmed:24761608]
Yao Xue Xue Bao. 2014 Feb;49(2):190-7.
Abnormal proliferation of vascular smooth muscle cells (VSMCs) plays an important role in several pathological processes of cardiovascular diseases. In this study, the effects of XCT790, a potent and selective inverse agonist of estrogen-related receptor alpha (ERRalpha), on rat VSMCs proliferation and related signal pathways were investigated. The proliferative activity of VSMCs was determined by CCK-8 assay. The mRNA levels of ERRalpha, PGC-1alpha, OPN and MCAD were assayed by RT-PCR. The protein levels of ERRalpha, ERK2 and p-ERK1/2 were evaluated by Western blotting. ELISA was used to assess the protein expression of VEGF. The results showed that XCT790 (5-20 micromol x L(-1)) inhibited rat VSMCs proliferation, and the expression of ERRalpha and its target genes, as well as p-ERK1/2, were also inhibited. XCT790 inhibited VSMCs proliferation in a dose-dependent manner at the dose range from 5 to 20 micromol x L(-1) and in a time-dependent manner at the dose range from 10 to 20 micromol x L(-1). These findings demonstrate that XCT790 inhibits rat VSMCs proliferation by down-regulating the gene level of ERRalpha and thus inhibiting the ERK signal pathway, suggesting that ERRalpha may be a novel potential target for therapeutic approaches to inhibit VSMCs proliferation, which plays an important role in several cardiovascular diseases.
Peroxisome proliferator-activated receptor gamma coactivator-1alpha enhances antiproliferative activity of 5'-deoxy-5-fluorouridine in cancer cells through induction of uridine phosphorylase.[Pubmed:19602572]
Mol Pharmacol. 2009 Oct;76(4):854-60.
Peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) is capable of coactivating several nuclear receptors and transcription factors that participate in the regulation of multiple metabolic processes, including gluconeogenesis, mitochondrial biogenesis, and adaptive thermogenesis. Uridine phosphorylase (UPase) catalyzes the reversible conversion of uridine into uracil and contributes to the antineoplastic activity of 5'-deoxy-5-fluorouridine (5'-DFUR) and homeostasis of uridine levels in plasma and tissues. This study demonstrates uridine phosphorylase as a novel target gene of PGC-1alpha, which induces the transcription and enzymatic activity of UPase in various cancer cells and thus augments their susceptibility to 5'-DFUR. PGC-1alpha-induced activation of UPase expression occurs at its transcription level that is mediated by an estrogen-related receptor (ERR) binding site (-1078 to -1070 base pairs) mapped in the promoter region of UPase gene. Our mutational studies using luciferase reporter construct together with electrophoretic mobility shift assays confirm the binding of ERR to PGC-1alpha-responsive element. Moreover, the inhibition of PGC-1alpha/ERRalpha-dependent signaling by 3-[4-(2,4-bis-trifluoromethylbenzyloxy)-3-methoxyphenyl]-2-cyano-N-(5-trifluorome thyl-1,3,4-thiadiazol-2-yl)acrylamide (XCT790) compromises the ability of PGC-1alpha to induce the transcript of UPase, indicating PGC-1alpha-dependent and ERRalpha-mediated up-regulation of UPase. Finally, the overexpression of PGC-1alpha sensitizes breast and colon cancer cells to growth inhibition by 5'-DFUR presumably by inducing apoptosis in tumor cells and XCT790 can inhibit the process. Taken together, our results corroborate the regulatory function of PGC-1alpha in uridine homeostasis and imply its links with the energy metabolism. The mechanistic elucidation of this association between both cellular pathways should advance the clinical use of 5-fluorouracil-based chemotherapy.


