MiglustatCAS# 72599-27-0 |
- Meprednisone
Catalog No.:BCC4893
CAS No.:1247-42-3
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Desonide
Catalog No.:BCC4967
CAS No.:638-94-8
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 72599-27-0 | SDF | Download SDF |
| PubChem ID | 51634 | Appearance | Powder |
| Formula | C10H21NO4 | M.Wt | 219.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | N-Butyldeoxynojirimycin; NB-DNJ; OGT918 | ||
| Solubility | Soluble in DMSO | ||
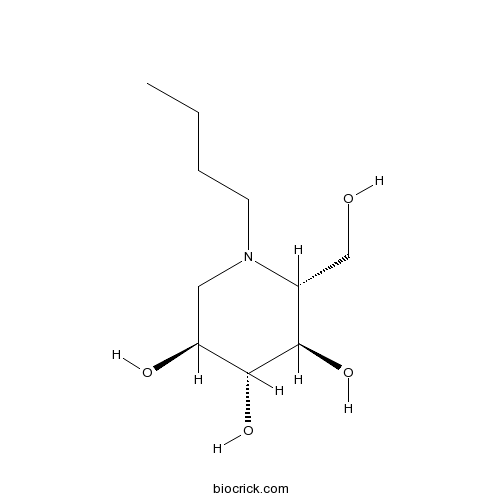
| Chemical Name | (2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol | ||
| SMILES | CCCCN1CC(C(C(C1CO)O)O)O | ||
| Standard InChIKey | UQRORFVVSGFNRO-UTINFBMNSA-N | ||
| Standard InChI | InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Miglustat(OGT918) is an inhibitor of glucosylceramide synthase, primarily to treat Type I Gaucher disease (GD1).
Target: Others
Miglustat is an inhibitor of the ceramide-specific glycosyltransferase, which catalyzes the first step of glycosphingolipid biosynthesis and is currently approved for the oral treatment of type 1 GD [1]. Consumption of a standard high-fat breakfast within 30 minutes before administration of miglustat significantly reduced peak exposure but did not significantly affect the extent of systemic exposure to miglustat. The peak plasma concentration (C(max)) decreased by 36% on average following administration with food. Area under the plasma concentration-time curve (AUC(0-infinity)) showed a modest (14%) decrease with food, but the 90% confidence interval was within the acceptance limit of 80% to 125%. The median (min-max) time to C(max) (t(max)) was prolonged from 2.5 (1.0-4.0) hours in the fasted state to 4.5 (1.5-8.0) hours in the fed state, whereas the apparent terminal half-life was approximately 8 hours and not affected by food [2]. References: | |||||

Miglustat Dilution Calculator

Miglustat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5604 mL | 22.8019 mL | 45.6038 mL | 91.2076 mL | 114.0095 mL |
| 5 mM | 0.9121 mL | 4.5604 mL | 9.1208 mL | 18.2415 mL | 22.8019 mL |
| 10 mM | 0.456 mL | 2.2802 mL | 4.5604 mL | 9.1208 mL | 11.4009 mL |
| 50 mM | 0.0912 mL | 0.456 mL | 0.9121 mL | 1.8242 mL | 2.2802 mL |
| 100 mM | 0.0456 mL | 0.228 mL | 0.456 mL | 0.9121 mL | 1.1401 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Miglustat(OGT918) is an inhibitor of glucosylceramide synthase, primarily to treat Type I Gaucher disease (GD1).
- Isosilybin
Catalog No.:BCN2406
CAS No.:72581-71-6
- Calebassine
Catalog No.:BCN2276
CAS No.:7257-29-6
- Angeloylgomisin Q
Catalog No.:BCN7033
CAS No.:72561-28-5
- Rifabutin
Catalog No.:BCC4936
CAS No.:72559-06-9
- Ceftazidime
Catalog No.:BCC5274
CAS No.:72558-82-8
- GIP (1-39)
Catalog No.:BCC5890
CAS No.:725474-97-5
- 6-chloro-9h-fluoren-2-amine
Catalog No.:BCC9231
CAS No.:7254-05-9
- Polygonal
Catalog No.:BCN4281
CAS No.:72537-20-3
- XCT790
Catalog No.:BCC5121
CAS No.:725247-18-7
- Paeonolide
Catalog No.:BCN2805
CAS No.:72520-92-4
- Specioside
Catalog No.:BCN4280
CAS No.:72514-90-0
- Lycobetaine
Catalog No.:BCN8313
CAS No.:72510-04-4
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- γ1-MSH
Catalog No.:BCC6021
CAS No.:72629-65-3
- Prosapogenin CP6
Catalog No.:BCN2535
CAS No.:72629-76-6
- Mollisorin A
Catalog No.:BCN7236
CAS No.:72704-04-2
- Ilicol
Catalog No.:BCN4282
CAS No.:72715-02-7
- H-D-Lys-OH.HCl
Catalog No.:BCC2989
CAS No.:7274-88-6
- Swainsonine
Catalog No.:BCC7602
CAS No.:72741-87-8
- 1-Ethoxycarbonyl-beta-carboline
Catalog No.:BCN3102
CAS No.:72755-19-2
- Odorine
Catalog No.:BCN4283
CAS No.:72755-20-5
- Odorinol
Catalog No.:BCN4284
CAS No.:72755-22-7
- Senecivernine
Catalog No.:BCN2135
CAS No.:72755-25-0
- β-Funaltrexamine hydrochloride
Catalog No.:BCC6850
CAS No.:72786-10-8
Preliminary Results on Long-Term Potentiation-Like Cortical Plasticity and Cholinergic Dysfunction After Miglustat Treatment in Niemann-Pick Disease Type C.[Pubmed:28092091]
JIMD Rep. 2017;36:19-27.
Niemann-Pick disease type C (NPC) is a rare autosomal recessive lysosomal storage disorder, which manifests clinically with a wide range of neurological signs and symptoms. We assessed multiple neurological, neuropsychological and neurophysiological biomarkers using a transcranial magnetic stimulation (TMS) multi-paradigm approach in two patients with NPC carrying a homozygous mutation in the NPC1 gene, and in two heterozygous family members.We assessed short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), long-interval intracortical inhibition (LICI), short-latency afferent inhibition (SAI) and long-term potentiation (LTP)-like cortical plasticity with a paired associative stimulation (PAS) protocol.Baseline SAI and LTP-like plasticity were impaired in both patients with NPC and in the symptomatic heterozygous NPC1 gene mutation carrier. Only a limited decrease in SICI and ICF was observed, while LICI was within normal range in all subjects at baseline. After 12 months of treatment with Miglustat, a considerable improvement in SAI and LTP-like plasticity was observed in both patients with NPC. In conclusion, these biomarkers could help to confirm the diagnosis of NPC, and may give an indication of prognostic outcomes in pharmacological trials.
Successful switch from enzyme replacement therapy to miglustat in an adult patient with type 1 Gaucher disease: a case report.[Pubmed:27821156]
J Med Case Rep. 2016 Nov 8;10(1):315.
BACKGROUND: Gaucher disease is one of the most common lipid-storage disorders, affecting approximately 1 in 75,000 births. Enzyme replacement therapy with recombinant glucocerebrosidase is currently considered the first-line treatment choice for patients with symptomatic Gaucher disease type 1. Oral substrate reduction therapy is generally considered a second-line treatment option for adult patients with mild to moderate Gaucher disease type 1 who are unable or unwilling to receive lifelong intravenous enzyme infusions. The efficacy and safety of the oral substrate reduction therapy Miglustat (Zavesca(R)) in patients with Gaucher disease type 1 have been established in both short-term clinical trials and long-term, open-label extension studies. Published data indicate that Miglustat can be used as maintenance therapy in patients with stable Gaucher disease type 1 switched from previous enzyme replacement therapy. CASE PRESENTATION: We report a case of a 44-year-old Caucasian man with Gaucher disease type 1 who was initially treated with enzyme replacement therapy but, owing to repeated cutaneous allergic reactions, had to be switched to Miglustat after several attempts with enzyme replacement therapy. Despite many attempts, desensitization treatment did not result in improved toleration of imiglucerase infusions, and the patient became unwilling to continue with any intravenous enzyme replacement therapy. He subsequently agreed to switch to oral substrate reduction therapy with Miglustat 100 mg twice daily titrated up to 100 mg three times daily over a short period. Long-term Miglustat treatment maintained both hemoglobin and platelet levels within acceptable ranges over 8 years. The patient's spleen volume decreased, his plasma chitotriosidase levels stayed at reduced levels, and his bone mineral density findings have remained stable throughout follow-up. The patient's quality of life has remained satisfactory. Miglustat showed good gastrointestinal tolerability in this patient, and no adverse events have been reported. CONCLUSIONS: Oral Miglustat therapy proved to be a valid alternative treatment to intravenous enzyme replacement therapy for long-term maintenance in this patient with Gaucher disease type 1, who showed persistent allergic intolerance to imiglucerase infusions. This report exemplifies the type of patient with Gaucher disease type 1 who can benefit from switching from enzyme replacement therapy to substrate reduction therapy.
Twelve years of experience with miglustat in the treatment of type 1 Gaucher disease: The Spanish ZAGAL project.[Pubmed:27836529]
Blood Cells Mol Dis. 2018 Feb;68:173-179.
We report data from a prospective, observational study (ZAGAL) evaluating Miglustat 100mg three times daily orally. in treatment-naive patients and patients with type 1 Gaucher Disease (GD1) switched from previous enzyme replacement therapy (ERT). Clinical evolution, changes in organ size, blood counts, disease biomarkers, bone marrow infiltration (S-MRI), bone mineral density by broadband ultrasound densitometry (BMD), safety and tolerability annual reports were analysed. Between May 2004 and April 2016, 63 patients received Miglustat therapy; 20 (32%) untreated and 43 (68%) switched. At the time of this report 39 patients (14 [36%] treatment-naive; 25 [64%] switch) remain on Miglustat. With over 12-year follow-up, hematologic counts, liver and spleen volumes remained stable. In total, 80% of patients achieved current GD1 therapeutic goals. Plasma chitotriosidase activity and CCL-18/PARC concentration showed a trend towards a slight increase. Reductions on S-MRI (p=0.042) with an increase in BMD (p<0.01) were registered. Gastrointestinal disturbances were reported in 25/63 (40%), causing Miglustat suspension in 11/63 (17.5%) cases. Thirty-eight patients (60%) experienced a fine hand tremor and two a reversible peripheral neuropathy. Overall, Miglustat was effective as a long-term therapy in mild to moderate naive and ERT stabilized patients. No unexpected safety signals were identified during 12-years follow-up.


