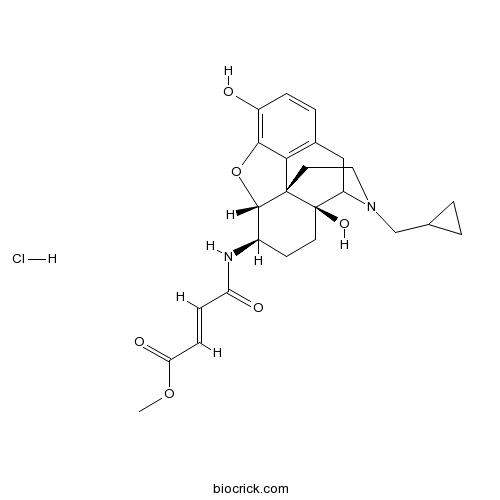
β-Funaltrexamine hydrochlorideCAS# 72786-10-8 |
- Bestatin trifluoroacetate
Catalog No.:BCC3909
CAS No.:223763-80-2
- Fumagillin
Catalog No.:BCC2347
CAS No.:23110-15-8
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Bestatin hydrochloride
Catalog No.:BCC3908
CAS No.:65391-42-6
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 72786-10-8 | SDF | Download SDF |
| PubChem ID | 6442032 | Appearance | Powder |
| Formula | C25H31ClN2O6 | M.Wt | 490.99 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | β-FNA | ||
| Solubility | Soluble to 20 mM in water with gentle warming and to 100 mM in DMSO | ||
| Chemical Name | methyl (E)-4-[[(4aS,7R,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-1,2,4,5,6,7,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl]amino]-4-oxobut-2-enoate;hydrochloride | ||
| SMILES | COC(=O)C=CC(=O)NC1CCC2(C3CC4=C5C2(C1OC5=C(C=C4)O)CCN3CC6CC6)O.Cl | ||
| Standard InChIKey | BIPHUOBUKMPSQR-ZWZZIDKESA-N | ||
| Standard InChI | InChI=1S/C25H30N2O6.ClH/c1-32-20(30)7-6-19(29)26-16-8-9-25(31)18-12-15-4-5-17(28)22-21(15)24(25,23(16)33-22)10-11-27(18)13-14-2-3-14;/h4-7,14,16,18,23,28,31H,2-3,8-13H2,1H3,(H,26,29);1H/b7-6+;/t16-,18?,23+,24+,25-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective μ opioid receptor antagonist. |

β-Funaltrexamine hydrochloride Dilution Calculator

β-Funaltrexamine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0367 mL | 10.1835 mL | 20.367 mL | 40.734 mL | 50.9175 mL |
| 5 mM | 0.4073 mL | 2.0367 mL | 4.0734 mL | 8.1468 mL | 10.1835 mL |
| 10 mM | 0.2037 mL | 1.0184 mL | 2.0367 mL | 4.0734 mL | 5.0918 mL |
| 50 mM | 0.0407 mL | 0.2037 mL | 0.4073 mL | 0.8147 mL | 1.0184 mL |
| 100 mM | 0.0204 mL | 0.1018 mL | 0.2037 mL | 0.4073 mL | 0.5092 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Senecivernine
Catalog No.:BCN2135
CAS No.:72755-25-0
- Odorinol
Catalog No.:BCN4284
CAS No.:72755-22-7
- Odorine
Catalog No.:BCN4283
CAS No.:72755-20-5
- 1-Ethoxycarbonyl-beta-carboline
Catalog No.:BCN3102
CAS No.:72755-19-2
- Swainsonine
Catalog No.:BCC7602
CAS No.:72741-87-8
- H-D-Lys-OH.HCl
Catalog No.:BCC2989
CAS No.:7274-88-6
- Ilicol
Catalog No.:BCN4282
CAS No.:72715-02-7
- Mollisorin A
Catalog No.:BCN7236
CAS No.:72704-04-2
- Prosapogenin CP6
Catalog No.:BCN2535
CAS No.:72629-76-6
- γ1-MSH
Catalog No.:BCC6021
CAS No.:72629-65-3
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
- Mesulergine hydrochloride
Catalog No.:BCC7139
CAS No.:72786-12-0
- ICI 118,551 hydrochloride
Catalog No.:BCC4029
CAS No.:72795-01-8
- Linderalactone
Catalog No.:BCN1251
CAS No.:728-61-0
- Estropipate
Catalog No.:BCC7719
CAS No.:7280-37-7
- Panaxydol
Catalog No.:BCN3701
CAS No.:72800-72-7
- OSI-930
Catalog No.:BCC1253
CAS No.:728033-96-3
- Atanine
Catalog No.:BCN3317
CAS No.:7282-19-1
- Deoxyartemisinin
Catalog No.:BCN4285
CAS No.:72826-63-2
- MIRA-1
Catalog No.:BCC2409
CAS No.:72835-26-8
- 1-Benzyl-5-phenylbarbituric acid
Catalog No.:BCC8461
CAS No.:72846-00-5
- Echitoveniline
Catalog No.:BCN7490
CAS No.:72855-79-9
- Crocandine
Catalog No.:BCN2070
CAS No.:72855-83-5
Effects of chronic administration of PL017 and beta-funaltrexamine hydrochloride on susceptibility of kainic acid-induced seizures in rats.[Pubmed:14985838]
Sheng Li Xue Bao. 2004 Feb 25;56(1):101-6.
There is evidence that 5-7 d after acute seizure episodes induced by kainic acid (KA) the rats develop a long-lasting increase in the susceptibility to seizures followed by spontaneous recurrent seizures (SRS). The present study was focused on the role of hippocampal mu opioid receptors (MORs) in the susceptibility of rats to seizures with the KA model of epilepsy. The rats received a convulsant dose of KA (10 mg/kg, i.p.) were continuously infused with a selective MOR agonist PL017 (2.09, 2.59, 3.29 microg/microl), or a selective MOR antagonist beta-funaltrexamine hydrochloride (beta-FNA, 0.88, 1.10, and 1.35 microg/microl) into ventral hippocampus by means of mini-osmotic pumps. Seven days later, the susceptibility of rats to seizures was checked by a subconvulsant dose of KA (5 mg/kg, i.p.). PL017 infusion shortened the latency and increased the stage of seizures induced by subconvulsant dose of KA in a dose-dependent manner. In contrast, infusion of beta-FNA exhibited a dose-dependent effect against seizures challenged by subconvulsant dose of KA. These results indicate that hippocampal MOR may exert a promoting effect on the susceptibility of rats to KA-induced seizures.
Mu antagonist and kappa agonist properties of beta-funaltrexamine (beta-FNA) in vivo: long-lasting spinal analgesia in mice.[Pubmed:2156986]
J Pharmacol Exp Ther. 1990 Mar;252(3):1006-11.
It is now well established that compounds classified as kappa opioids can, in circumstances where they produce no measurable agonist effects, antagonize the actions of mu opioids. Largely on the basis of studies in vitro, beta-funaltrexamine (beta-FNA) has been classified as a reversible kappa agonist and long acting mu antagonist. The present study investigated the possibility that the mu antagonist profile of this compound could be related to its kappa agonist actions. We used two tests of analgesia (the acetic acid writhing test and the hot-water tail-flick test) and selective kappa agonists and antagonists given at supraspinal and spinal sites in mice. Intrathecal (i.t.) administration of beta-FNA, but not the selective kappa agonist U50,488H, produced long-lasting and dose-related analgesia in the writhing test for periods up to 48 hr after a single dose. In contrast, i.t. beta-FNA had no agonist actions in the tail-flick test. The kappa antagonist, nor-binaltorphimine (nor-BNI) produced no agonist effects in either analgesic test when given i.t. In the writhing test, nor-BNI produced a rightward displacement of the beta-FNA dose-response line regardless of whether beta-FNA was given 10 min or 4 hr before testing, indicating that i.t. beta-FNA was acting as a kappa agonist in this test. As both i.t. morphine and beta-FNA are active in the writhing test, the antagonist actions of i.t. beta-FNA could be evaluated only in the tail-flick test. beta-FNA, but not nor-BNI, blocked the effects of i.t. morphine in the tail-flick test.(ABSTRACT TRUNCATED AT 250 WORDS)
The irreversible narcotic antagonistic and reversible agonistic properties of the fumaramate methyl ester derivative of naltrexone.[Pubmed:6263637]
Eur J Pharmacol. 1981 Apr 9;70(4):445-51.
The fumaramate methyl ester derivatives of naltrexone (beta-FNA) and oxymorphone (beta-FOA) were both found to be reversible agonists on the guinea pig ileal longitudinal muscle preparation. In addition, beta-FNA possessed on irreversible antagonistic effect against morphine whereas beta-FOA had no such capacity. Analysis by pA2 values revealed that beta-FOA resembled pure agonists like morphine and enkephalin while beta-FNA resembled the mixed agonist-antagonists like nalorphine and pentazocine. The antagonism by beta-FNA was very selective in that it antagonized pure agonists but had little or no effect on the effects of either mixed agonist-antagonists, ethylketocyclazocine or other non-opiate-type agonists like norepinephrine.


