Tosedostat (CHR2797)Aminopeptidase inhibitor CAS# 238750-77-1 |
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- VS-5584 (SB2343)
Catalog No.:BCC2047
CAS No.:1246560-33-7
- XL388
Catalog No.:BCC2059
CAS No.:1251156-08-7
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 238750-77-1 | SDF | Download SDF |
| PubChem ID | 15547703 | Appearance | Powder |
| Formula | C21H30N2O6 | M.Wt | 406.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tosedostat;CHR-2797; CHR 2797;CHR2797 | ||
| Solubility | DMSO : 25 mg/mL (61.51 mM; Need ultrasonic and warming) | ||
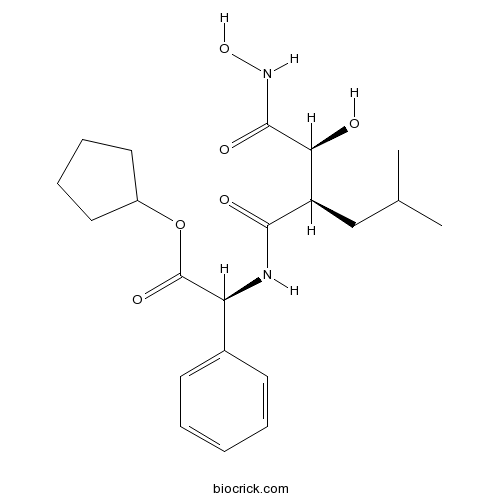
| Chemical Name | cyclopentyl (2S)-2-[[(2R)-2-[(1S)-1-hydroxy-2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoyl]amino]-2-phenylacetate | ||
| SMILES | CC(C)CC(C(C(=O)NO)O)C(=O)NC(C1=CC=CC=C1)C(=O)OC2CCCC2 | ||
| Standard InChIKey | FWFGIHPGRQZWIW-SQNIBIBYSA-N | ||
| Standard InChI | InChI=1S/C21H30N2O6/c1-13(2)12-16(18(24)20(26)23-28)19(25)22-17(14-8-4-3-5-9-14)21(27)29-15-10-6-7-11-15/h3-5,8-9,13,15-18,24,28H,6-7,10-12H2,1-2H3,(H,22,25)(H,23,26)/t16-,17+,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aminopeptidase inhibitor (IC50 values are 100, 150, 220, > 1000, > 5000, > 10000 and > 30000 nM for LAP, PuSA, aminopeptidase N, aminopeptidase B, PILSAP, LTA4 hydrolase and MetAP2 respectively). Potently inhibits tumor cell proliferation in a variety of tumor cell lines in vitro and in vivo. |

Tosedostat (CHR2797) Dilution Calculator

Tosedostat (CHR2797) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4602 mL | 12.301 mL | 24.6021 mL | 49.2041 mL | 61.5052 mL |
| 5 mM | 0.492 mL | 2.4602 mL | 4.9204 mL | 9.8408 mL | 12.301 mL |
| 10 mM | 0.246 mL | 1.2301 mL | 2.4602 mL | 4.9204 mL | 6.1505 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.492 mL | 0.9841 mL | 1.2301 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.492 mL | 0.6151 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tosedostat is a novel and potent oral aminopeptidase inhibitor with clinical activity in a previous phase 1–2 study in elderly patients with relapsed or refractory acute myeloid leukaemia (AML). [2]
Aminopeptidases play a key role in the protein cell cycle. Inhibition of aminopeptidase results in the amino acid deprivation response, which occurs selectively in transformed cells and leads to upregulation of proapoptotic factors including CHOP and NOXA, activation of stress-related pathways such as NFκB, and inhibition of mTOR, which switches off protein synthesis. [2]
Tosedostat (CHR-2797) is converted intracellularly into a pharmacologically active metabolite CHR-79888. [1]
Tosedostat has antiproliferative, antiangiogenic and proapoptotic effects. Tosedostat is currently in a clinical trial phase for anticancer therapy, and displayed a broad antifungal activity against different Candida spp, including Candida glabrata. Tosedostat depletes sensitive tumour cells of amino acids by blocking protein recycling and thereby generates an antiproliferative effect. Tosedostat has activity in older patients with relapsed or refractory AML. [2]
References:
1.Van Herpen CM, Eskens FA, de Jonge M et al. A Phase Ib dose-escalation study to evaluate safety and tolerability of the addition of the aminopeptidase inhibitor tosedostat (CHR-2797) to paclitaxel in patients with advanced solid tumours. Br J Cancer. 2010 Oct 26;103(9):1362-8.
2.Cortes J, Feldman E, Yee K et al. Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): a randomised open-label phase 2 study. Lancet Oncol. 2013 Apr;14(4):354-62.
- Ethyl2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
Catalog No.:BCC8979
CAS No.:238749-50-3
- Liensinine perchlorate
Catalog No.:BCN6335
CAS No.:2385-63-9
- Ambroxol HCl
Catalog No.:BCC5067
CAS No.:23828-92-4
- Ac-Trp-OEt
Catalog No.:BCC3110
CAS No.:2382-80-1
- Trichokaurin
Catalog No.:BCN4851
CAS No.:23811-50-9
- Hinesol
Catalog No.:BCC9232
CAS No.:23811-08-7
- Pogostone
Catalog No.:BCN2696
CAS No.:23800-56-8
- 6-Hydroxyindole
Catalog No.:BCN8310
CAS No.:2380-86-1
- Homovanillyl alcohol
Catalog No.:BCN7173
CAS No.:2380-78-1
- 4-Aminopyrazolo[3,4-d]pyrimidine
Catalog No.:BCC8690
CAS No.:2380-63-4
- Methylprednisolone Sodium Succinate
Catalog No.:BCC5629
CAS No.:2375-03-3
- Rivulobirin E
Catalog No.:BCN5090
CAS No.:237407-59-9
- Zapotinin
Catalog No.:BCC9192
CAS No.:14813-20-8
- Boc-Lys(Z)-OH
Catalog No.:BCC2722
CAS No.:2389-45-9
- H-Lys(Boc)-OMe.HCl
Catalog No.:BCC2983
CAS No.:2389-48-2
- Z-Lys(Boc)-OH
Catalog No.:BCC2763
CAS No.:2389-60-8
- Dexamethasone dipropionate
Catalog No.:BCC8934
CAS No.:55541-30-5
- Alphaxalone
Catalog No.:BCC7545
CAS No.:23930-19-0
- 5-(2-Aminopropyl)-7-cyanoindolin-1-yl)propyl benzoate
Catalog No.:BCC8718
CAS No.:239463-72-0
- 5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile (2R,3R)-2,3-dihydroxybutanedioate
Catalog No.:BCN1479
CAS No.:239463-85-5
- Glochidonol
Catalog No.:BCN5091
CAS No.:23963-54-4
- 4,4'-Bis(5-methyl-2-benzoxazolyl)stilbene
Catalog No.:BCC8657
CAS No.:2397-00-4
- Meranzin
Catalog No.:BCN5092
CAS No.:23971-42-8
- Delta-9-Tetrahydrocannabinolic acid
Catalog No.:BCN8098
CAS No.:23978-85-0
X-ray crystal structures of an orally available aminopeptidase inhibitor, Tosedostat, bound to anti-malarial drug targets PfA-M1 and PfA-M17.[Pubmed:25645579]
Proteins. 2015 Apr;83(4):789-95.
New anti-malarial treatments are desperately required to face the spread of drug resistant parasites. Inhibition of metalloaminopeptidases, PfA-M1 and PfA-M17, is a validated therapeutic strategy for treatment of Plasmodium falciparum malaria. Here, we describe the crystal structures of PfA-M1 and PfA-M17 bound to chemotherapeutic agent Tosedostat. The inhibitor occupies the enzymes' putative product egress channels in addition to the substrate binding pockets; however, adopts different binding poses when bound to PfA-M1 and PfA-M17. These findings will be valuable for the continued development of selective inhibitors of PfA-M1 and PfA-M17.
Phase II study of tosedostat with cytarabine or decitabine in newly diagnosed older patients with acute myeloid leukaemia or high-risk MDS.[Pubmed:26568032]
Br J Haematol. 2016 Jan;172(2):238-45.
Tosedostat, an oral aminopeptidase inhibitor, has synergy with cytarabine and hypomethylating agents. We performed a Phase II trial to determine rates of complete remission (CR) and survival using tosedostat with cytarabine or decitabine in older patients with untreated acute myeloid leukaemia (AML) or high-risk myelodysplastic syndrome (MDS). Thirty-four patients >/=60 years old (median age 70 years; range, 60-83) were randomized to receive tosedostat (120 mg on days 1-21 or 180 mg continuously) with 5 d of either cytarabine (1 g/m2 /d) or decitabine (20 mg/m2 /d) every 35 d. Twenty-nine patients (85%) had AML, including 15 (44%) with secondary AML/MDS, and 5 (15%) had MDS-refractory anaemia with excess blasts type 2. The CR/CR with incomplete count recovery (CRi) rate was 53% [9 in each arm; 14 CR (41%) and 4 CRi (12%)], attained in 6 of 14 patients with adverse cytogenetics and 4 of 7 with FLT3-internal tandem duplication mutations. Median follow-up was 11.2 months (range, 0.5-22.3), and median survival was 11.5 months (95% confidence interval, 5.2-16.7). Twenty-three patients (67.6%) were treated as outpatients and 10 of these patients required hospitalization for febrile neutropenia. No Grade 3-4 non-haematological toxicities required withdrawal from study. Tosedostat with cytarabine or decitabine is tolerated in older patients with untreated AML/MDS, results in a CR/CRi rate of >50%, and warrants further study in larger trials.
Update on rational targeted therapy in AML.[Pubmed:26972558]
Blood Rev. 2016 Jul;30(4):275-83.
Acute myeloid leukemia (AML) remains a challenge to both patients and clinicians. Despite improvements in our understanding of the disease, treatment has changed minimally and outcomes remain poor for the majority of patients. Within the last decade, there have been an increasing number of potential targets and pathways identified for development in AML. The classes of agents described in this review include but are not limited to epigenetic modifiers such as IDH inhibitors, BET inhibitors, and HDAC inhibitors as well as cell cycle and signaling inhibitors such as Aurora kinase inhibitors and CDK inhibitors. While the developments are encouraging, it is unlikely that targeting a single pathway will result in long-term disease control. Accordingly, we will also highlight potential rational partners for the novel agents described herein.
Emerging therapies for acute myeloid leukemia.[Pubmed:28420416]
J Hematol Oncol. 2017 Apr 18;10(1):93.
Acute myeloid leukemia (AML) is characterized by clinical and biological heterogeneity. Despite the advances in our understanding of its pathobiology, the chemotherapy-directed management has remained largely unchanged in the past 40 years. However, various novel agents have demonstrated clinical activity, either as single agents (e.g., isocitrate dehydrogenase (IDH) inhibitors, vadastuximab) or in combination with standard induction/consolidation at diagnosis and with salvage regimens at relapse. The classes of agents described in this review include novel cytotoxic chemotherapies (CPX-351 and vosaroxin), epigenetic modifiers (guadecitabine, IDH inhibitors, histone deacetylase (HDAC) inhibitors, bromodomain and extraterminal (BET) inhibitors), FMS-like tyrosine kinase receptor 3 (FLT3) inhibitors, and antibody-drug conjugates (vadastuximab), as well as cell cycle inhibitors (volasertib), B-cell lymphoma 2 (BCL-2) inhibitors, and aminopeptidase inhibitors. These agents are actively undergoing clinical investigation alone or in combination with available chemotherapy.
In Vitro Radiosensitization of Esophageal Cancer Cells with the Aminopeptidase Inhibitor CHR-2797.[Pubmed:26291737]
Radiat Res. 2015 Sep;184(3):259-65.
With the increased incidence of esophageal cancer, chemoradiotherapy continues to play an important role in the management of this disease. Developing potent radiosensitizers is therefore critical for improving outcomes. The use of drugs that have already undergone clinical testing is an appealing approach once the side effects and tolerated doses are established. Here, we demonstrate that the aminopeptidase inhibitor, CHR-2797/tosedostat, increases the radiosensitivity of esophageal cancer cell lines (FLO-1 and OE21) in vitro in both normoxic and physiologically relevant low oxygen conditions. To our knowledge, the effective combination of CHR-2797 with radiation exposure has not been reported previously in any cancer cell type. The mechanism of increased radiosensitivity was not dependent on the induction of DNA damage or DNA repair kinetics. Our data support the need for further preclinical testing of CHR-2797 in combination with radiotherapy for the treatment of esophageal cancer.
Aminopeptidase inhibition as a targeted treatment strategy in myeloma.[Pubmed:19372548]
Mol Cancer Ther. 2009 Apr;8(4):762-70.
Myeloma cells are highly dependent on the unfolded protein response to assemble folded immunoglobulins correctly. Therefore, targeting protein handling within a myeloma cell by inhibiting the aminopeptidase enzyme system, which catalyses the hydrolysis of amino acids from the proteins NH2 terminus, represents a therapeutic approach. CHR-2797, a novel aminopeptidase inhibitor, is able to inhibit proliferation and induce growth arrest and apoptosis in myeloma cells, including cells resistant to conventional chemotherapeutics. It causes minimal inhibition of bone marrow stromal cell (BMSC) proliferation but is able to overcome the microenvironmental protective effects, inhibiting the proliferation of myeloma cells bound to BMSCs and the increase in vascular endothelial growth factor levels seen when myeloma cells and BMSCs are bound together. Additive and synergistic effects are seen with bortezomib, melphalan, and dexamethasone. Apoptosis occurs via both caspase-dependent and non-caspase-dependent pathways with an increase in Noxa, cleavage of Mcl-1, and activation of the unfolded protein response. Autophagy is also seen. CHR-2797 causes an up-regulation of genes involved in the proteasome/ubiquitin pathway, as well as aminopeptidases, and amino acid deprivation response genes. In conclusion, inhibiting protein turnover using the aminopeptidase inhibitor CHR-2797 results in myeloma cell apoptosis and represents a novel therapeutic approach that warrants further investigation in the clinical setting.
CHR-2797: an antiproliferative aminopeptidase inhibitor that leads to amino acid deprivation in human leukemic cells.[Pubmed:18701491]
Cancer Res. 2008 Aug 15;68(16):6669-79.
CHR-2797 is a novel metalloenzyme inhibitor that is converted into a pharmacologically active acid product (CHR-79888) inside cells. CHR-79888 is a potent inhibitor of a number of intracellular aminopeptidases, including leucine aminopeptidase. CHR-2797 exerts antiproliferative effects against a range of tumor cell lines in vitro and in vivo and shows selectivity for transformed over nontransformed cells. Its antiproliferative effects are at least 300 times more potent than the prototypical aminopeptidase inhibitor, bestatin. However, the mechanism by which inhibition of these enzymes leads to proliferative changes is not understood. Gene expression microarrays were used to profile changes in mRNA expression levels in the human promyelocytic leukemia cell line HL-60 treated with CHR-2797. This analysis showed that CHR-2797 treatment induced a transcriptional response indicative of amino acid depletion, the amino acid deprivation response, which involves up-regulation of amino acid synthetic genes, transporters, and tRNA synthetases. These changes were confirmed in other leukemic cell lines sensitive to the antiproliferative effects of CHR-2797. Furthermore, CHR-2797 treatment inhibited phosphorylation of mTOR substrates and reduced protein synthesis in HL-60 cells, both also indicative of amino acid depletion. Treatment with CHR-2797 led to an increase in the concentration of intracellular small peptides, the substrates of aminopeptidases. It is suggested that aminopeptidase inhibitors, such as CHR-2797 and bestatin, deplete sensitive tumor cells of amino acids by blocking protein recycling, and this generates an antiproliferative effect. CHR-2797 is orally bioavailable and currently undergoing phase II clinical investigation in the treatment of myeloid leukemia.


