AlphaxaloneDirect activator and potentiator of GABAA CAS# 23930-19-0 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Hypaconine
Catalog No.:BCN8640
CAS No.:63238-68-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23930-19-0 | SDF | Download SDF |
| PubChem ID | 104845 | Appearance | Powder |
| Formula | C21H32O3 | M.Wt | 332.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
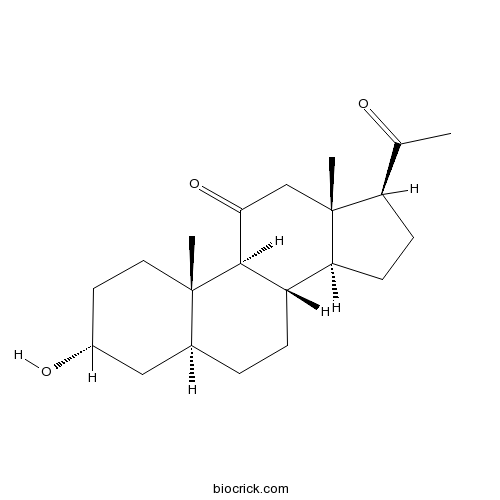
| Chemical Name | (3R,5S,8S,9S,10S,13S,14S,17S)-17-acetyl-3-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-11-one | ||
| SMILES | CC(=O)C1CCC2C1(CC(=O)C3C2CCC4C3(CCC(C4)O)C)C | ||
| Standard InChIKey | DUHUCHOQIDJXAT-OLVMNOGESA-N | ||
| Standard InChI | InChI=1S/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13-,14+,15-,16+,17-,19+,20-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A neurosteroid anesthetic that directly activates and potentiates GABAA receptor-activated membrane current (IGABA). Efficacy but not potency is determined by the alpha subunit of the receptor (EC50 values are 1.4, 1.8, 2.1, 2.4 and 2.5 μM for α1β1γ3, α1β1γ1, β1γ1, α2β1γ2L and α1β1γ2L isoforms respectively). |

Alphaxalone Dilution Calculator

Alphaxalone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0077 mL | 15.0385 mL | 30.077 mL | 60.154 mL | 75.1925 mL |
| 5 mM | 0.6015 mL | 3.0077 mL | 6.0154 mL | 12.0308 mL | 15.0385 mL |
| 10 mM | 0.3008 mL | 1.5038 mL | 3.0077 mL | 6.0154 mL | 7.5192 mL |
| 50 mM | 0.0602 mL | 0.3008 mL | 0.6015 mL | 1.2031 mL | 1.5038 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3008 mL | 0.6015 mL | 0.7519 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dexamethasone dipropionate
Catalog No.:BCC8934
CAS No.:55541-30-5
- Z-Lys(Boc)-OH
Catalog No.:BCC2763
CAS No.:2389-60-8
- H-Lys(Boc)-OMe.HCl
Catalog No.:BCC2983
CAS No.:2389-48-2
- Boc-Lys(Z)-OH
Catalog No.:BCC2722
CAS No.:2389-45-9
- Zapotinin
Catalog No.:BCC9192
CAS No.:14813-20-8
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Ethyl2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
Catalog No.:BCC8979
CAS No.:238749-50-3
- Liensinine perchlorate
Catalog No.:BCN6335
CAS No.:2385-63-9
- Ambroxol HCl
Catalog No.:BCC5067
CAS No.:23828-92-4
- Ac-Trp-OEt
Catalog No.:BCC3110
CAS No.:2382-80-1
- Trichokaurin
Catalog No.:BCN4851
CAS No.:23811-50-9
- Hinesol
Catalog No.:BCC9232
CAS No.:23811-08-7
- 5-(2-Aminopropyl)-7-cyanoindolin-1-yl)propyl benzoate
Catalog No.:BCC8718
CAS No.:239463-72-0
- 5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile (2R,3R)-2,3-dihydroxybutanedioate
Catalog No.:BCN1479
CAS No.:239463-85-5
- Glochidonol
Catalog No.:BCN5091
CAS No.:23963-54-4
- 4,4'-Bis(5-methyl-2-benzoxazolyl)stilbene
Catalog No.:BCC8657
CAS No.:2397-00-4
- Meranzin
Catalog No.:BCN5092
CAS No.:23971-42-8
- Delta-9-Tetrahydrocannabinolic acid
Catalog No.:BCN8098
CAS No.:23978-85-0
- Tolnaftate
Catalog No.:BCC4869
CAS No.:2398-96-1
- 13-Deacetyltaxachitriene A
Catalog No.:BCN7390
CAS No.:239800-99-8
- Dehydroepiandrosterone enanthate
Catalog No.:BCC8930
CAS No.:23983-43-9
- Pyrocatechol monoglucoside
Catalog No.:BCN4667
CAS No.:2400-71-7
- QX 314 bromide
Catalog No.:BCC6889
CAS No.:24003-58-5
- Griffithazanone A
Catalog No.:BCN4813
CAS No.:240122-30-9
Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of gamma-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone.[Pubmed:24328079]
J Med Chem. 2014 Jan 9;57(1):171-90.
A model of the alignment of neurosteroids and ent-neurosteroids at the same binding site on gamma-aminobutyric acid type A (GABAA) receptors was evaluated for its ability to identify the structural features in ent-neurosteroids that enhance their activity as positive allosteric modulators of this receptor. Structural features that were identified included: (1) a ketone group at position C-16, (2) an axial 4alpha-OMe group, and (3) a C-18 methyl group. Two ent-steroids were identified that were more potent than the anesthetic steroid Alphaxalone in their threshold for and duration of loss of the righting reflex in mice. In tadpoles, loss of righting reflex for these two ent-steroids occurs with EC50 values similar to those found for allopregnanolone. The results indicate that ent-steroids have considerable potential to be developed as anesthetic agents and as drugs to treat brain disorders that are ameliorated by positive allosteric modulators of GABAA receptor function.
The use of injectable alphaxalone as a single agent and in combination with ketamine, xylazine, and morphine in the Chilean rose tarantula, Grammostola rosea.[Pubmed:25632665]
J Zoo Wildl Med. 2014 Dec;45(4):792-801.
This study evaluated the use of the injectable anesthetic, Alphaxalone, as a single agent and in combination with ketamine, xylazine, and morphine in the Chilean rose tarantula, Grammostola rosea. Between two and four animals were evaluated for each anesthetic protocol, and two unanesthetized animals were evaluated for comparative purposes. Anesthetic duration, depth, and quality were assessed by scoring responses to tactile and trichobothria stimulation, muscle tone, purposeful movement, righting response, and heart rate throughout each anesthetic event. Alphaxalone administered into the dorsal opisthosoma in the location of the heart at 200 mg/kg produced moderate anesthetic effect with a median duration of 28 min (n = 3; range 25-50). A combination of 200 mg/kg of Alphaxalone and 20 mg/kg of ketamine induced a deep anesthetic state with a median anesthetic duration of 27 min (n = 4; range 16-42). The combination of 200 mg/kg of Alphaxalone and 20 mg/kg of xylazine produced deep anesthesia with a median duration of 70 min (n = 4; range 37-207). Morphine administered at 5 mg/kg 30 min prior to injection with 200 mg/kg Alphaxalone had anesthetic durations of 9 and 30 min (n = 2). Heartbeats could not be detected for periods of 7-27 min following anesthetic induction for the majority of animals receiving the Alphaxalone/ketamine and Alphaxalone/xylazine anesthetic combinations. No mortality was associated with any of the anesthetic protocols used; however, ambient temperature and ecdysis were identified as important factors that may alter response to anesthetics in these animals.
Alphaxalone Reformulated: A Water-Soluble Intravenous Anesthetic Preparation in Sulfobutyl-Ether-beta-Cyclodextrin.[Pubmed:25517195]
Anesth Analg. 2015 May;120(5):1025-31.
BACKGROUND: Alphaxalone is a neuroactive steroid anesthetic that is poorly water soluble. It was formulated in 1972 as Althesin(R) using Cremophor(R) EL, a nonionic surfactant additive. The product was a versatile short-acting IV anesthetic used in clinical practice in many countries from 1972 to 1984. It was withdrawn from clinical practice because of hypersensitivity to Cremophor EL. In the investigations reported here, we compared the properties of 3 anesthetics: a new aqueous solution of Alphaxalone dissolved in 7-sulfobutyl-ether-beta-cyclodextrin (SBECD, a water-soluble molecule with a lipophilic cavity that enables drug solubilization in water); a Cremophor EL preparation of Alphaxalone; and propofol. METHODS: Two solutions of Alphaxalone (10 mg/mL) were prepared: one using 13% w/v solution of SBECD in 0.9% saline (PHAX) and the other a solution of Alphaxalone prepared as described in the literature using 20% Cremophor EL (ALTH). A solution of propofol (10 mg/mL; PROP) in 10% v/v soya bean oil emulsion was used as a comparator anesthetic. Jugular IV catheters were implanted in male Wistar rats (180-220 g) under halothane anesthesia. Separate groups of 10 implanted rats each were given IV injections of PHAX, ALTH, or PROP from 1.2 mg/kg to lethal doses. Doses of each drug that caused anesthesia (loss of righting reflex and response to tail pinch) and lethality in 50% of rats were calculated by probit analysis. The drugs were also compared for effects on arterial blood pressure and heart rate. RESULTS: IV PHAX, ALTH, and PROP caused dose-related sedation and anesthesia, with 50% effective dose (ED50) values for loss of righting reflex being 2.8, 3.0, and 4.6 mg/kg, respectively. PROP led to death in 10 of 10 rats at doses >30 mg/kg (50% lethal dose (LD50) = 27.7 mg/kg). A dose of Alphaxalone 53 mg/kg as ALTH caused 10 of 10 rats to die (LD50 = 43.6 mg/kg), whereas none died when given the same doses of Alphaxalone formulated in SBECD. PHAX caused 20% lethality at the maximal dose tested of 84 mg/kg. PHAX caused less cardiovascular depression than PROP. Control experiments with the 3 drug-free vehicles showed no effects. CONCLUSIONS: Alphaxalone caused fast-onset anesthesia at the same dose for both formulations (PHAX and ALTH). The use of SBECD as a drug-solubilizing excipient did not alter the anesthetic effect of Alphaxalone, but it did increase the therapeutic index of Alphaxalone in PHAX compared with ALTH. PHAX has a higher safety margin than the propofol lipid formulation and also the Alphaxalone formulation in Cremophor EL (ALTH).
A Phase 1c Trial Comparing the Efficacy and Safety of a New Aqueous Formulation of Alphaxalone with Propofol.[Pubmed:26226029]
Anesth Analg. 2015 Oct;121(4):914-24.
BACKGROUND: Phaxan (PHAX, Chemic Labs, Canton, MA) is an aqueous solution of 10 mg/mL Alphaxalone and 13% 7-sulfobutylether beta-cyclodextrin (betadex). In preclinical studies, PHAX is a fast onset-offset IV anesthetic like propofol, but causes less cardiovascular depression. This first-in-man study was designed to find the anesthetic dose of PHAX and to compare it with an equivalent dose of propofol for safety, efficacy, and quality of recovery from anesthesia and sedation. METHODS: The study adhered to compliance with Good Clinical Practices regulations (clinical trials registry number, ACTRN12611000343909). This randomized, double-blind study compared PHAX and propofol using a Bayesian algorithm to determine dose equivalence for effects on the bispectral index (BIS). Male volunteers, ASA physical status I, gave written informed consent (n = 12 per group; PHAX or propofol). Parameters assessed for 80 minutes after drug injection (single bolus dose) were pain on injection, involuntary movement, BIS, blood pressure, need for airway support, and, as measures of recovery from sedation, the Richmond Agitation and Sedation Scale and the Digit Symbol Substitution Test. Arterial blood was withdrawn for biochemistry, hematology, and complement levels. RESULTS: No subject complained of pain on injection with PHAX, whereas 8 of the 12 subjects given propofol did. Nine PHAX and 8 propofol subjects reached BIS values of
The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse.[Pubmed:19228970]
J Neurosci. 2009 Feb 18;29(7):2177-87.
The GABA(A) receptor has been identified as the single most important target for the intravenous anesthetic propofol. How effects at this receptor are then translated into a loss of consciousness, however, remains a mystery. One possibility is that anesthetics act on natural sleep pathways. Here, we test this hypothesis by exploring the anesthetic sensitivities of GABAergic synaptic currents in three specific brain nuclei that are known to be involved in sleep. Using whole-cell electrophysiology, we have recorded GABAergic IPSCs from the tuberomammillary nucleus (TMN), the perifornical area (Pef), and the locus ceruleus (LC) in brain slices from both wild-type mice and mice that carry a specific mutation in the GABA(A) receptor beta(3) subunit (N265M), which greatly reduces their sensitivity to propofol, but not to the neurosteroid Alphaxalone. We find that this in vivo pattern of anesthetic sensitivity is mirrored in the hypothalamic TMN and Pef nuclei, consistent with their role as direct anesthetic targets. In contrast, anesthetic sensitivity in the LC was unaffected by the beta(3)N265M mutation, ruling out this nucleus as a major target for propofol. In support of the hypothesis that orexinergic neurons in the Pef are involved in propofol anesthesia, we further show that these neurons are selectively inhibited by GABAergic drugs in vivo during anesthesia, and that a modulation in the activity of Pef neurons alone can affect loss of righting reflex. Overall, our results support the idea that GABAergic anesthetics such as propofol exert their effects, at least in part, by modulating hypothalamic sleep pathways.
Auto-modulation of neuroactive steroids on GABA A receptors: a novel pharmacological effect.[Pubmed:17084864]
Neuropharmacology. 2007 Feb;52(2):672-83.
GABA(A) receptor function is modulated by various important drugs including neuroactive steroids that act on allosteric modulatory sites and can directly activate GABA(A) receptor channels at high concentrations. We used whole cell patch-clamp recordings and rapid applications of the neuroactive steroid Alphaxalone to investigate repetitive steroid effects. Alphaxalone potentiation of submaximal GABA-evoked currents was enhanced significantly by repetitive coapplications at all investigated recombinant isoforms (alpha1beta3delta, alpha1beta3gamma2L, alpha6beta3delta, alpha6beta3gamma2L) and at GABA(A) receptors of differentiated human NT2 neurons. A similar increase of current amplitudes was induced by repetitive applications of a high steroid concentration without GABA. We refer to these reversible effects as auto-modulation because repeated interactions of steroids enhanced their own pharmacological impact at the receptor sites in a time and concentration dependent manner without affecting GABA controls. Pronounced auto-modulatory actions were also measured using the neurosteroid 5alpha-THDOC in contrast to indiplon, THIP, and pentobarbital indicating a steroid specificity. Protein kinase A inhibition significantly reduced Alphaxalone auto-modulation at alpha1beta3gamma2L, alpha6beta3gamma2L, and alpha6beta3delta subtypes while it enhanced potentiation at alpha1beta3delta isoforms suggesting a crucial influence of receptor subunit composition and phosphorylation for steroid actions. Especially at extrasynaptic GABA(A) receptor sites containing the delta subunit steroid auto-modulation may have a critical role in enhancing potentiation of GABA-induced currents.
Subunit dependent modulation of GABAA receptor function by neuroactive steroids.[Pubmed:10082863]
Brain Res. 1999 Feb 20;819(1-2):75-82.
Neurosteroids are potent, endogenous modulators of GABAA receptor function in the central nervous system. The endogenous progesterone metabolite allopregnanolone (ALP) and the synthetic steroid compound Alphaxalone (AFX) have been shown to both directly activate and potentiate GABAA receptor-activated membrane current (IGABA). The role of different alpha and gamma subunit subtypes in modulation of IGABA by ALP and AFX was investigated using recombinant GABAA receptor isoforms expressed in Xenopus oocytes. Changing or removal of the alpha subunit subtype altered the efficacy of both ALP and AFX (alpha2beta1gamma2L>alpha1beta1gamma2L>>beta1gamma2L) to potentiate IGABA, but did not alter the potency of the neuroactive steroids at these receptor isoforms. The efficacy of ALP to enhance IGABA was also dependent on the gamma subunit subtype (alpha1beta1gamma3>alpha1beta1gamma2L = alpha1beta1gamma1). AFX also had higher efficacy in the alpha1beta1gamma3 receptor isoform compared to alpha1beta1gamma1. In contrast to ALP, the potency of AFX was greater in the alpha1beta1gamma3 and alpha1beta1gamma1 receptor isoforms compared to alpha1beta1gamma2L. This study provides evidence that the alpha subunit subtype determines the efficacy, but not the potency, of these neuroactive steroids to potentiate IGABA. The gamma3 subunit subtype increases the maximal efficacy of neuroactive steroids compared to other gamma subunit subtypes. These results suggest that the heteromeric assembly of different GABAA receptor isoforms containing different subunit subtypes results in multiple steroid recognition sites on GABAA receptors that in turn produce distinctly different modulatory interactions between neuroactive steroids acting at the GABAA receptor.


