HinesolCAS# 23811-08-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23811-08-7 | SDF | Download SDF |
| PubChem ID | 161437 | Appearance | Powder |
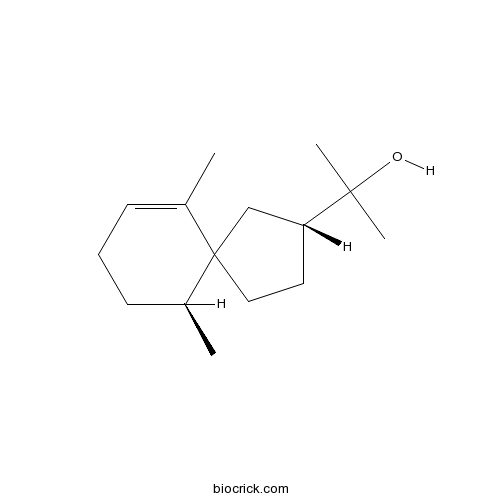
| Formula | C15H26O | M.Wt | 222.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(3R,6S)-6,10-dimethylspiro[4.5]dec-9-en-3-yl]propan-2-ol | ||
| SMILES | CC1CCC=C(C12CCC(C2)C(C)(C)O)C | ||
| Standard InChIKey | ICWHTQRTTHCUHW-IKCIUXDWSA-N | ||
| Standard InChI | InChI=1S/C15H26O/c1-11-6-5-7-12(2)15(11)9-8-13(10-15)14(3,4)16/h6,12-13,16H,5,7-10H2,1-4H3/t12-,13+,15?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hinesol Dilution Calculator

Hinesol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4964 mL | 22.482 mL | 44.964 mL | 89.9281 mL | 112.4101 mL |
| 5 mM | 0.8993 mL | 4.4964 mL | 8.9928 mL | 17.9856 mL | 22.482 mL |
| 10 mM | 0.4496 mL | 2.2482 mL | 4.4964 mL | 8.9928 mL | 11.241 mL |
| 50 mM | 0.0899 mL | 0.4496 mL | 0.8993 mL | 1.7986 mL | 2.2482 mL |
| 100 mM | 0.045 mL | 0.2248 mL | 0.4496 mL | 0.8993 mL | 1.1241 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pogostone
Catalog No.:BCN2696
CAS No.:23800-56-8
- 6-Hydroxyindole
Catalog No.:BCN8310
CAS No.:2380-86-1
- Homovanillyl alcohol
Catalog No.:BCN7173
CAS No.:2380-78-1
- 4-Aminopyrazolo[3,4-d]pyrimidine
Catalog No.:BCC8690
CAS No.:2380-63-4
- Methylprednisolone Sodium Succinate
Catalog No.:BCC5629
CAS No.:2375-03-3
- Rivulobirin E
Catalog No.:BCN5090
CAS No.:237407-59-9
- 4-Amino-3-hydroxybenzoic acid
Catalog No.:BCC8681
CAS No.:2374-03-0
- trans-3'-O-Benzoyl-4'-O-methylkhellactone
Catalog No.:BCN6921
CAS No.:23733-95-1
- trans-Methylkhellactone
Catalog No.:BCN6919
CAS No.:23733-92-8
- 6-Hydroxy-4-Methylcoumarin
Catalog No.:BCC9206
CAS No.:2373-31-1
- Chebulic acid
Catalog No.:BCN3260
CAS No.:23725-05-5
- Nardosinone
Catalog No.:BCN2324
CAS No.:23720-80-1
- Trichokaurin
Catalog No.:BCN4851
CAS No.:23811-50-9
- Ac-Trp-OEt
Catalog No.:BCC3110
CAS No.:2382-80-1
- Ambroxol HCl
Catalog No.:BCC5067
CAS No.:23828-92-4
- Liensinine perchlorate
Catalog No.:BCN6335
CAS No.:2385-63-9
- Ethyl2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
Catalog No.:BCC8979
CAS No.:238749-50-3
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Zapotinin
Catalog No.:BCC9192
CAS No.:14813-20-8
- Boc-Lys(Z)-OH
Catalog No.:BCC2722
CAS No.:2389-45-9
- H-Lys(Boc)-OMe.HCl
Catalog No.:BCC2983
CAS No.:2389-48-2
- Z-Lys(Boc)-OH
Catalog No.:BCC2763
CAS No.:2389-60-8
- Dexamethasone dipropionate
Catalog No.:BCC8934
CAS No.:55541-30-5
- Alphaxalone
Catalog No.:BCC7545
CAS No.:23930-19-0
Chemotype diversity of Psidium guajava L.[Pubmed:29913321]
Phytochemistry. 2018 Sep;153:129-137.
The essential oil of Psidium guajava L. has been studied for pharmacological and industrial purposes, without considering the plant's genotype regarding the heterogeneity of its composition. The present study aimed to characterize the chemotype diversity of the essential oil extracted from the leaves of 22 genotypes of P. guajava grown in two different environments in the state of Espirito Santo, Brazil, and to identify the different chemical markers present in these plants. Essential oil from the leaves of the P. guajava genotypes was extracted by hydrodistillation, and its chemical composition was analyzed by gas chromatography-flame ionization detection (GC-FID) and gas chromatography-mass spectrometry (GC-MS). Thirty-three compounds were identified, comprising 87.5-99.0% of the total composition, with a prevalence of sesquiterpenes in all samples. The major compounds identified consisted of (E)-trans-Caryophyllene, alpha-Humulene, trans-Nerolidol, beta-Bisabolene, beta-Bisabolol, and Hinesol, the first of which was identified as a possible chemical marker for the species. Multivariate factor analysis of the chemical composition of P. guajava oil identified three chemotypes: Commercial - PAL, SEC, PS, PET, C7, C11, and C17MI, characterized by high levels of beta-Selinene, alpha-Selinene, Hinesol, and 14-hydroxy-epi-(E)-caryophyllene, with beta-Selinene and alpha-Selinene as the chemical markers; C10 and C13, exhibiting high levels of Elemol, trans-Nerolidol, trans-beta-Eudesmol, and (2Z, 6Z)-Farnesol, which were indicated as chemical markers, and Cortibel - C1, C2, C3, C4, C5, C6, C8, C9, C12, C14, C15, C16, C17LI, which retained high levels of alpha-Cedrene, cis-alpha-Bergamotene, alpha-Humulene, Humulene epoxide, epi-alpha-Cadinol, beta-Bisabolol, and alpha-Bisabolol, with beta-Bisabolol and alpha-Bisabolol as the chemical markers. The use of guava genotypes with different chemotypes, that are agronomically favorable to fruit production and essential oil exploitation adds value to the crop and renders it more sustainable. Given guava crops produce large amounts of leaf biomass, resulting from successive prunings, the extraction of their essential oil, which retains commercially valuable compounds, can be feasible.
Hinesol, a compound isolated from the essential oils of Atractylodes lancea rhizome, inhibits cell growth and induces apoptosis in human leukemia HL-60 cells.[Pubmed:25833731]
J Nat Med. 2015 Jul;69(3):332-9.
Hinesol is a unique sesquiterpenoid isolated from the Chinese traditional medicine, Atractylodes lancea rhizome. In a previous study, we screened various natural products in human leukemia HL-60 cells and identified an essential oil fraction from A. lancea rhizome that exhibited apoptosis-inducing activity in these cells; Hinesol was subsequently shown to be the compound responsible for this apoptosis-inducing activity. In this study, we describe the cytotoxic effects and molecular mechanisms of Hinesol in HL-60 cells. The antitumor effect of Hinesol was associated with apoptosis. When HL-60 cells were treated with Hinesol, characteristic features of apoptosis, such as nuclear fragmentation and DNA fragmentation, were observed. These growth-inhibitory and apoptosis-inducing activities of Hinesol in leukemia cells were much stronger than those of beta-eudesmol, another compound isolated from the essential oil fraction. Furthermore, Hinesol induced activation of c-Jun N-terminal kinase (JNK), but not p38, prior to the onset of apoptosis. These results suggested that Hinesol induced apoptosis through the JNK signaling pathway in HL-60 cells. Therefore, Hinesol may represent a novel medicinal drug having indications in the treatment of various cancers, including leukemia.


