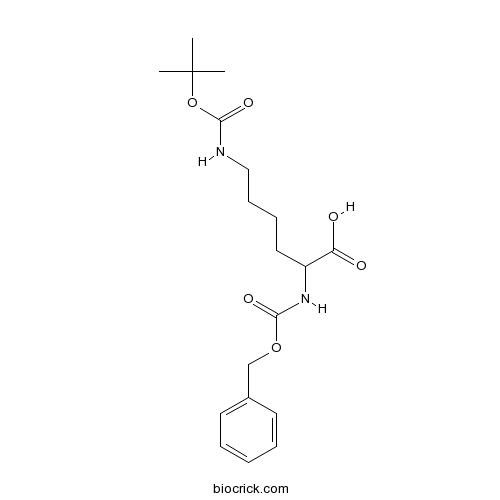
Z-Lys(Boc)-OHCAS# 2389-60-8 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2389-60-8 | SDF | Download SDF |
| PubChem ID | 294900 | Appearance | Powder |
| Formula | C19H28N2O6 | M.Wt | 380.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-[(2-methylpropan-2-yl)oxycarbonylamino]-2-(phenylmethoxycarbonylamino)hexanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NCCCCC(C(=O)O)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | DYSBKEOCHROEGX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H28N2O6/c1-19(2,3)27-17(24)20-12-8-7-11-15(16(22)23)21-18(25)26-13-14-9-5-4-6-10-14/h4-6,9-10,15H,7-8,11-13H2,1-3H3,(H,20,24)(H,21,25)(H,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Lys(Boc)-OH Dilution Calculator

Z-Lys(Boc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6288 mL | 13.1441 mL | 26.2881 mL | 52.5762 mL | 65.7203 mL |
| 5 mM | 0.5258 mL | 2.6288 mL | 5.2576 mL | 10.5152 mL | 13.1441 mL |
| 10 mM | 0.2629 mL | 1.3144 mL | 2.6288 mL | 5.2576 mL | 6.572 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5258 mL | 1.0515 mL | 1.3144 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2629 mL | 0.5258 mL | 0.6572 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Lys(Boc)-OH
- H-Lys(Boc)-OMe.HCl
Catalog No.:BCC2983
CAS No.:2389-48-2
- Boc-Lys(Z)-OH
Catalog No.:BCC2722
CAS No.:2389-45-9
- Zapotinin
Catalog No.:BCC9192
CAS No.:14813-20-8
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Ethyl2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
Catalog No.:BCC8979
CAS No.:238749-50-3
- Liensinine perchlorate
Catalog No.:BCN6335
CAS No.:2385-63-9
- Ambroxol HCl
Catalog No.:BCC5067
CAS No.:23828-92-4
- Ac-Trp-OEt
Catalog No.:BCC3110
CAS No.:2382-80-1
- Trichokaurin
Catalog No.:BCN4851
CAS No.:23811-50-9
- Hinesol
Catalog No.:BCC9232
CAS No.:23811-08-7
- Pogostone
Catalog No.:BCN2696
CAS No.:23800-56-8
- 6-Hydroxyindole
Catalog No.:BCN8310
CAS No.:2380-86-1
- Dexamethasone dipropionate
Catalog No.:BCC8934
CAS No.:55541-30-5
- Alphaxalone
Catalog No.:BCC7545
CAS No.:23930-19-0
- 5-(2-Aminopropyl)-7-cyanoindolin-1-yl)propyl benzoate
Catalog No.:BCC8718
CAS No.:239463-72-0
- 5-[(2R)-2-Aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-1H-indole-7-carbonitrile (2R,3R)-2,3-dihydroxybutanedioate
Catalog No.:BCN1479
CAS No.:239463-85-5
- Glochidonol
Catalog No.:BCN5091
CAS No.:23963-54-4
- 4,4'-Bis(5-methyl-2-benzoxazolyl)stilbene
Catalog No.:BCC8657
CAS No.:2397-00-4
- Meranzin
Catalog No.:BCN5092
CAS No.:23971-42-8
- Delta-9-Tetrahydrocannabinolic acid
Catalog No.:BCN8098
CAS No.:23978-85-0
- Tolnaftate
Catalog No.:BCC4869
CAS No.:2398-96-1
- 13-Deacetyltaxachitriene A
Catalog No.:BCN7390
CAS No.:239800-99-8
- Dehydroepiandrosterone enanthate
Catalog No.:BCC8930
CAS No.:23983-43-9
- Pyrocatechol monoglucoside
Catalog No.:BCN4667
CAS No.:2400-71-7
Radical acylation of L-lysine derivatives and L-lysine-containing peptides by peroxynitrite-treated diacetyl and methylglyoxal.[Pubmed:24328571]
Free Radic Res. 2014 Mar;48(3):357-70.
Highly electrophilic alpha-dicarbonyls such as diacetyl, methylglyoxal, 3-deoxyglucosone, and4,5-dioxovaleric acid have been characterized as secondary catabolites that can aggregate proteins and form DNA nucleobase adducts in several human maladies, including Alzheimer's disease, rheumatoid arthritis, diabetes, sepsis, renal failure, and respiratory distress syndrome. In vitro, diacetyl and methylglyoxal have also been shown to rapidly add up the peroxynitrite anion (k2 ~ 10(4)-10(5) M(-1) s(-1)), a potent biological nucleophile, oxidant and nitrosating agent, followed by carbon chain cleavage to carboxylic acids via acetyl radical intermediate that can modify amino acids. In this study, we used the amino acid derivatives Ac-Lys-OMe and Z-Lys-OMe and synthesized the tetrapeptides H-KALA-OH, Ac-KALA-OH, and H-K(Boc)ALA-OH to reveal the preferential Lys amino group targeted by acyl radical generated by the alpha-dicarbonyl/peroxynitrite system. The pH profiles of the reactions are bell-shaped, peaking at approximately 7.5; hence, they are close to the pKa values of ONOOH and of the catalytic H2PO4(-) anion. RP-HPLC and ESI-MS analyses of reaction products confirmed (alpha)N- and ()N-acetylation of Lys by diacetyl as well as acetylation and formylation by methylglyoxal, with preference for the alpha-amino group. These data suggest the possibility of radical acylation of proteins in epigenetic processes, where enzymatic acetylation of these biomolecules is a well-documented event, recently reported to be as critical to the cell cycle as phosphorylation. Also noteworthy is the observed formylation of L-Lys containing peptides by methylglyoxal never reported to occur in amino acid residues of peptides and proteins.


