Bestatin hydrochlorideCAS# 65391-42-6 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65391-42-6 | SDF | Download SDF |
| PubChem ID | 11957481 | Appearance | Powder |
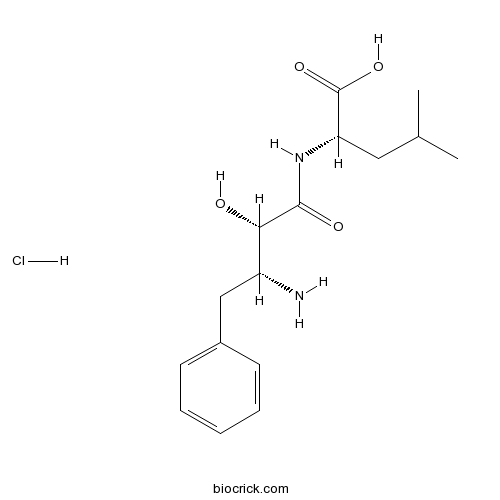
| Formula | C16H25ClN2O4 | M.Wt | 344.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ubenimex hydrochloride | ||
| Solubility | ≥125mg/mL in DMSO | ||
| Chemical Name | (2S)-2-[[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic acid;hydrochloride | ||
| SMILES | CC(C)CC(C(=O)O)NC(=O)C(C(CC1=CC=CC=C1)N)O.Cl | ||
| Standard InChIKey | XGDFITZJGKUSDK-UDYGKFQRSA-N | ||
| Standard InChI | InChI=1S/C16H24N2O4.ClH/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11;/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22);1H/t12-,13+,14+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bestatin hydrochloride is an inhibitor of CD13 (Aminopeptidase N)/APN and leukotriene A4 hydrolase, used for cancer treatment.In Vitro:Bestatin enhances ATRA-induced differentiation and inhibits ATRA-driven phosphorylation of p38 MAPK in ATRA-sensitive APL NB4 cells. Bestatin can not reverse the differentiation block in ATRA-resistant APL MR2 cells. CD13 ligation with anti-CD13 antibody WM-15 results in phosphorylation of p38 MAPK, reduces the inhibition of Bestatin on the phosphorylation of p38 MAPK, and completely abolishes the enhancement of Bestatin on ATRA-inducing differentiation in NB4 cells[2]. Bestatin (600 μM)-treated cells progress slower through the cell cycle due to decreased rate of cell growth and the frequency of cell division. Bestatin inhibits the frequency of mitosis and the inherent multinuclearity in D. discoideum, and is not cytotoxic to D. discoideum cells at 0-600 μM. Bestatin inhibits aminopeptidase activity in lysates of PsaA-GFP- and GFP-expressing cells by 69.39% ± 10.5% and 39.93% ± 18.7% of control, respectively[4].In Vivo:Bestatin (20 μM) significantly reduces CD13 expression in diabetic mice and results a significant inhibition of MMP-9 specific gelationolytic band densities compared to diabetic vehicle-treated mice. Bestatin treatment significantly inhibits the expression of VEGF and heparanase in diabetic mice. Intravitreal bestatin treatment significantly downregulates the expression of both HIF-1α and VEGF in diabetic mice retinas. Furthermore, the upregulated expression of heparanase in diabetic mice retinas is significantly inhibited by intravitreal bestatin treatment[1]. Bestatin (10, 1, and 0.1mg/kg, i.p.) treatment before the antigen-potentiated humoral response to SRBC results in an increased number of splenocytes producing hemolytic anti-SRBC antibodies (PFC) and the 2-ME-resistant serum hemagglutinin titer (at a dose of 0.1 mg/kg). Bestatin (1 and 0.1 mg/kg) administered to mice five times on alternate days after cyclophosphamide injection does not change the suppressive effect of the drug regarding the number of PFC, and even causes the further decrease of the total anti-SRBC hemagglutinins at dose of 1 mg/kg on day 7 after antigen stimulation[3]. References: | |||||

Bestatin hydrochloride Dilution Calculator

Bestatin hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9 mL | 14.4999 mL | 28.9998 mL | 57.9996 mL | 72.4995 mL |
| 5 mM | 0.58 mL | 2.9 mL | 5.8 mL | 11.5999 mL | 14.4999 mL |
| 10 mM | 0.29 mL | 1.45 mL | 2.9 mL | 5.8 mL | 7.2499 mL |
| 50 mM | 0.058 mL | 0.29 mL | 0.58 mL | 1.16 mL | 1.45 mL |
| 100 mM | 0.029 mL | 0.145 mL | 0.29 mL | 0.58 mL | 0.725 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bestatin hydrochloride (Ubenimex ) is a competitive aminopeptidase inhibitor, which is studied for use in the treatment of acute myelocytic leukemia.
- alpha-Isowighteone
Catalog No.:BCN4197
CAS No.:65388-03-6
- D-Chicoric Acid
Catalog No.:BCC8148
CAS No.:52248-48-3
- H-D-Pro-OMe.HCl
Catalog No.:BCC3025
CAS No.:65365-28-8
- 2,6-Tropanediol
Catalog No.:BCN1875
CAS No.:65356-02-7
- Demethylvestitol
Catalog No.:BCN4079
CAS No.:65332-45-8
- Scopolamine hydrobromide trihydrate
Catalog No.:BCC8188
CAS No.:6533-68-2
- L-Norgestrel
Catalog No.:BCC9105
CAS No.:797-64-8
- Primulic Acid 1
Catalog No.:BCC8236
CAS No.:65312-86-9
- Calhex 231 hydrochloride
Catalog No.:BCC7931
CAS No.:652973-93-8
- Ketoconazole
Catalog No.:BCC2272
CAS No.:65277-42-1
- Arvenin II
Catalog No.:BCN7878
CAS No.:65247-28-1
- Cucurbitacin B 2-O-beta-D-glucoside
Catalog No.:BCN3119
CAS No.:65247-27-0
- Morin hydrate
Catalog No.:BCC8214
CAS No.:654055-01-3
- Altholactone
Catalog No.:BCN4786
CAS No.:65408-91-5
- H-D-Asp-Ome
Catalog No.:BCC2896
CAS No.:65414-78-0
- Boc-Asn(Xan)-OH
Catalog No.:BCC3361
CAS No.:65420-40-8
- DL-Dab.2HCl
Catalog No.:BCC2669
CAS No.:65427-54-5
- 1-Methoxyphaseollidin
Catalog No.:BCN7185
CAS No.:65428-13-9
- Phebalosin
Catalog No.:BCN4198
CAS No.:6545-99-9
- Acantrifoic acid A
Catalog No.:BCN6488
CAS No.:654663-85-1
- Fmoc-Asn(Trt)-ol
Catalog No.:BCC2585
CAS No.:654670-89-0
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Sitagliptin phosphate
Catalog No.:BCC9148
CAS No.:654671-78-0
- (Z)-23-Coumaroylhederagenin
Catalog No.:BCN3748
CAS No.:654678-61-2
Exploration of Sitagliptin as a potential inhibitor for the M1 Alanine aminopeptidase enzyme in Plasmodium falciparum using computational docking.[Pubmed:23559748]
Bioinformation. 2013;9(6):293-8.
Plasmodium falciparum has limited capacity for de novo amino acid synthesis and rely on degradation of host hemoglobin to maintain protein metabolism and synthesis of proteins. M1 alanine aminopeptidase enzyme of the parasite involved in the terminal degradation of host hemoglobin was subjected to in silico screening with low molecular weight protease inhibitors. The km (avg) of the enzyme M1 alanine aminopeptidase for the substrate DL - Alanine beta Napthylamide Hydrochloride was estimated as 322.05microM. The molecular interactions between the enzyme and the substrate and the mechanism of enzyme action were analyzed which paved way for inhibition strategies. Among all the inhibitors screened, Sitagliptin was found to be most potent inhibitor with ki of 0.152 microM in its best orientation whereas the ki(avg) was 2.0055 microM. The ki of Sitagliptin is lower than the km of M1 alanine aminopeptidase for the substrate DL - Alanine beta Napthylamide Hydrochloride (322.05 microM) and Ki of the known inhibitor Bestatin. Therefore Sitagliptin may serve as a potent competitive inhibitor of the enzyme M1 alanine aminopeptidase of Plasmodium falciparum.
Central penetration and stability of N-terminal tripeptide of insulin-like growth factor-I, glycine-proline-glutamate in adult rat.[Pubmed:15752541]
Neuropeptides. 2005 Apr;39(2):81-7.
Insulin-like growth factor-I is a neurotrophic factor and can prevent neurons from ischemic brain injury. However, the large molecular weight and metabolic effects can be problematic in its central delivery. Glycine-proline-glutamate (GPE) is the N-terminal tripeptide of insulin-like growth factor-I, which is naturally cleaved in the plasma and brain tissues. GPE reduces neuronal loss from hypoxic-ischemic brain injury following central administration. Central penetration and the stability of GPE in the plasma and central nervous system were examined in rats using radioimmunoassay and HPLC. GPE was rapidly metabolised in the plasma (8 min) after intraperitoneal administration. Despite having a short half-life in plasma, GPE was detected in the cerebrospinal fluid up to 40 min after intraperitoneal administration. With present of peptidase inhibitors, GPE existed in the brain tissue up to 3 h after intracerebroventricular administration, suggesting a role for peptolysis in its stability. The endopeptidase inhibitors 4- (2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) reduced GPE metabolism in the brain tissue while acid peptidase inhibitor pepstatin-A decreased GPE metabolism in the plasma. GPE reduced neuronal loss in the CA1-2 sub-region of the hippocampus given (intraperitoneally) after 30 min of hypoxic-ischemic injury in adult rats, further suggested the effectiveness of GPE central uptake. These results indicated that GPE crosses the blood-CSF and the functional CSF-brain barriers. The longer half-life of GPE in the CNS may be due to its unique enzymatic stability.
Enhancement effect of P-gp inhibitors on the intestinal absorption and antiproliferative activity of bestatin.[Pubmed:23981338]
Eur J Pharm Sci. 2013 Nov 20;50(3-4):420-8.
Bestatin is an immunomodulator with antitumor activity. This study was performed to investigate the effect of P-gp on the intestinal absorption and antiproliferative activity of bestatin. Our results showed that P-gp inhibitors significantly increased rat intestinal absorption of bestatin in vivo and in vitro. The net efflux ratio of bestatin was 2.2 across mock-/MDR1-MDCK cell monolayers and was decreased by P-gp inhibitors, indicating bestatin was a substrate of P-gp. Furthermore, the IC50 values of bestatin on U937 and K562 cells were decreased dramatically and the intracellular concentrations of bestatin were increased by incubation of cells with verapamil or Cyclosporin A. K562/ADR cells exhibited a higher IC50 value and a lower intracellular level of bestatin. The bestatin level in K562/ADR cells was partially restored by incubation with doxorubicin. However, P-gp and APN mRNA levels were not changed by bestatin. These results suggested that the intestinal absorption and accumulation in cancer cells for bestatin were limited by P-gp-mediated efflux. Additional attention should be paid to the alternative exposure of bestatin when bestatin was coadministered with drugs as P-gp substrates in clinic.
Asymmetric nitroaldol reaction. Synthesis of taxotere side chain and (-)-bestatin using (1R)-8-phenylmenthyl glyoxylate.[Pubmed:15074936]
J Org Chem. 2004 Apr 16;69(8):2844-50.
The nitroaldol reaction of (1R)-8-phenylmenthyl glyoxylate (3b) with 1-nitro-1-phenylmethane (4) or with 1-nitro-2-phenylethane (13) led stereoselectively to adducts syn-2b and syn-12b, which were then transformed into the Taxotere side chain and (-)-Bestatin hydrochloride in overall yields of 52% and 31%, respectively.
Purification and characterization of hatching enzyme from brine shrimp Artemia salina.[Pubmed:20119628]
Acta Biochim Biophys Sin (Shanghai). 2010 Feb;42(2):165-71.
By using Artemia chorion as a specific substrate, the hatching enzyme from Artemia salina (AHE) was purified by gel-filtration and ion-exchange chromatography, and characterized biochemically and enzymatically in this study. It was found that the AHE had a molecular weight of 82.2 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and often contained 73.3 kDa molecules in preparation. The AHE had obvious choriolytic activity, which was optimal at pH 7.0 and a temperature of 408C. The Km value of the AHE for dimethyl casein was 8.20 mg/ml. The AHE activity was almost completely inhibited by soybean trypsin inhibitor and p-amidinophenyl methane sulfonyl fluoride hydrochloride, greatly inhibited by N-tosyl-L-lysyl chloromethyl ketone, phenylmethanesulfonyl fluoride, and lima bean trypsin inhibitor, slightly inhibited by pepstatin, N-tosyl-L-phenylalanyl chloromethyl ketone, leupeptin, N-ethylmaleimide, and iodoacetamide, and not inhibited by chymostatin and bestatin. All these results imply that AHE is most probably a trypsin-type serine protease. Besides of these, AHE was also sensitive to EDTA and Zn21. Combined with the results that the EDTA-pre-treated HE activity could be perfectly recovered by Zn21, it is indicated that AHE might be also a kind of Zn-metalloprotease.


