KetoconazoleInhibitor of cyclosporine oxidase and testosterone 6 beta-hydroxylase CAS# 65277-42-1 |
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- Alizarin
Catalog No.:BCN3479
CAS No.:72-48-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65277-42-1 | SDF | Download SDF |
| PubChem ID | 47576 | Appearance | Powder |
| Formula | C26H28Cl2N4O4 | M.Wt | 531.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (±)-Ketoconazol | ||
| Solubility | DMSO : 25 mg/mL (47.04 mM; Need ultrasonic) | ||
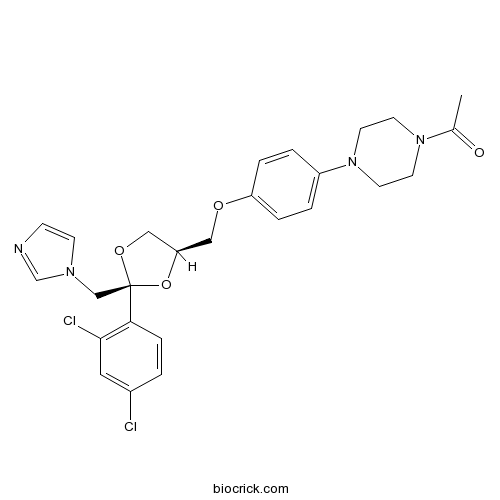
| Chemical Name | 1-[4-[4-[[(2S,4R)-2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone | ||
| SMILES | CC(=O)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl | ||
| Standard InChIKey | XMAYWYJOQHXEEK-ZEQKJWHPSA-N | ||
| Standard InChI | InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of cytochrome P450c17. Enhances the formation of myelin basic protein-positive oligodendrocytes from oligodendrocyte progenitor cells in vitro and promotes remyelination in vivo. Also an antifungal agent. Brain penetrant. |

Ketoconazole Dilution Calculator

Ketoconazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8817 mL | 9.4086 mL | 18.8172 mL | 37.6343 mL | 47.0429 mL |
| 5 mM | 0.3763 mL | 1.8817 mL | 3.7634 mL | 7.5269 mL | 9.4086 mL |
| 10 mM | 0.1882 mL | 0.9409 mL | 1.8817 mL | 3.7634 mL | 4.7043 mL |
| 50 mM | 0.0376 mL | 0.1882 mL | 0.3763 mL | 0.7527 mL | 0.9409 mL |
| 100 mM | 0.0188 mL | 0.0941 mL | 0.1882 mL | 0.3763 mL | 0.4704 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ketoconazole inhibits cyclosporine oxidase and testosterone 6 beta-hydroxylase with IC50 of 0.19 mM and 0.22 mM, respectively.
- Arvenin II
Catalog No.:BCN7878
CAS No.:65247-28-1
- Cucurbitacin B 2-O-beta-D-glucoside
Catalog No.:BCN3119
CAS No.:65247-27-0
- Dehydrocrenatidine
Catalog No.:BCN4196
CAS No.:65236-62-6
- N-Glyceryltaurine
Catalog No.:BCN1753
CAS No.:65222-42-6
- Gossypin
Catalog No.:BCN7987
CAS No.:652-78-8
- Isosorbide
Catalog No.:BCC4667
CAS No.:652-67-5
- Avermectin B1b
Catalog No.:BCC1383
CAS No.:65195-56-4
- Avermectin B1a
Catalog No.:BCC1382
CAS No.:65195-55-3
- (16R)-E-Isositsirikine
Catalog No.:BCN4000
CAS No.:6519-27-3
- (16R)-Dihydrositsirikine
Catalog No.:BCN4195
CAS No.:6519-26-2
- 4'-Hydroxyflavanone
Catalog No.:BCN6548
CAS No.:6515-37-3
- 7-Hydroxyflavanone
Catalog No.:BCN6539
CAS No.:6515-36-2
- Calhex 231 hydrochloride
Catalog No.:BCC7931
CAS No.:652973-93-8
- Primulic Acid 1
Catalog No.:BCC8236
CAS No.:65312-86-9
- L-Norgestrel
Catalog No.:BCC9105
CAS No.:797-64-8
- Scopolamine hydrobromide trihydrate
Catalog No.:BCC8188
CAS No.:6533-68-2
- Demethylvestitol
Catalog No.:BCN4079
CAS No.:65332-45-8
- 2,6-Tropanediol
Catalog No.:BCN1875
CAS No.:65356-02-7
- H-D-Pro-OMe.HCl
Catalog No.:BCC3025
CAS No.:65365-28-8
- D-Chicoric Acid
Catalog No.:BCC8148
CAS No.:52248-48-3
- alpha-Isowighteone
Catalog No.:BCN4197
CAS No.:65388-03-6
- Bestatin hydrochloride
Catalog No.:BCC3908
CAS No.:65391-42-6
- Morin hydrate
Catalog No.:BCC8214
CAS No.:654055-01-3
- Altholactone
Catalog No.:BCN4786
CAS No.:65408-91-5
A successful case of pregnancy in a woman with ACTH-independent Cushing's syndrome treated with ketoconazole and metyrapone.[Pubmed:28277127]
Gynecol Endocrinol. 2017 May;33(5):349-352.
Cushing's syndrome (CS) is a rare disease caused by a chronic excess of cortisol. Hypercortisolaemia may affect reproductive system leading to infertility in women. However, some of the patients remain fertile, although pregnancy is uncommon. In our report, we describe the case of a 31-years old woman suffering from hypertension, oligomenorrhea, easy bruising, muscle weakness and elevated levels of cortisol. During hospitalization, high level of serum cortisol with stiff diurnal rhythm and undetectable plasma ACTH concentration were found. The overnight 1 mg dexamethasone (DEX) suppression test and the test with 8 mg of DEX were performed - plasma cortisol levels after both doses of DEX were over expected values. Thus, the diagnosis of ACTH independent hypercortisolaemia was established. After three weeks of Ketoconazole treatment, high level of beta-HCG was found corresponding to the third week of pregnancy. The Ketoconazole was shift to metyrapone but afterwards Ketoconazole was added again. The treatment was well tolerated and pregnancy proceeded without complications. US scan revealed a 2 cm adenoma of the left adrenal gland, confirmed by CT. An adrenalectomy was performed. Concluding, we think that medical treatment of CS in pregnant women is well tolerated and safe both for the mother and fetus.
A potential in situ gel formulation loaded with novel fabricated poly(lactide-co-glycolide) nanoparticles for enhancing and sustaining the ophthalmic delivery of ketoconazole.[Pubmed:28331311]
Int J Nanomedicine. 2017 Mar 8;12:1863-1875.
Oral Ketoconazole therapy is commonly associated with serious hepatotoxicity. Improving ocular drug delivery could be sufficient to treat eye fungal infections. The purpose of this study was to develop optimized Ketoconazole poly(lactide-co-glycolide) nanoparticles (NPs) with subsequent loading into in situ gel (ISG) formulation for ophthalmic drug delivery. Three formulation factors were optimized for their effect on particle size (Y1) and entrapment efficiency (Y2) utilizing central composite experimental design. Interaction among components was studied using differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) spectroscopy. Ketoconazole crystalline state was studied using X-ray powder diffraction. Six different polymeric ISG formulations were prepared and loaded with either optimized NPs or a pure drug. The prepared ISG formulations were characterized for in vitro gelation, drug release and antifungal activity. The permeation through human epithelial cell line was also investigated. The results revealed that all the studied formulation parameters significantly affected Y1 and Y2 of the developed NPs. DSC and FTIR studies illustrated compatibility among NP components, while there was a change from the crystalline state to the amorphous state of the NPs. The in vitro release from the ISG formulations loaded with drug NPs showed sustained and enhanced drug release compared to pure drug formulations. In addition, ISG loaded with NPs showed enhanced anti-fungal activity compared to pure drug formulations. Alginate-chitosan ISG formulation loaded with optimized Ketoconazole NPs illustrated higher drug permeation through epithelial cell lines and is considered as an effective ophthalmic drug delivery in the treatment of fungal eye infections.
Revisiting the Metabolism and Bioactivation of Ketoconazole in Human and Mouse Using Liquid Chromatography-Mass Spectrometry-Based Metabolomics.[Pubmed:28335386]
Int J Mol Sci. 2017 Mar 13;18(3). pii: ijms18030621.
Although Ketoconazole (KCZ) has been used worldwide for 30 years, its metabolic characteristics are poorly described. Moreover, the hepatotoxicity of KCZ limits its therapeutic use. In this study, we used liquid chromatography-mass spectrometry-based metabolomics to evaluate the metabolic profile of KCZ in mouse and human and identify the mechanisms underlying its hepatotoxicity. A total of 28 metabolites of KCZ, 11 of which were novel, were identified in this study. Newly identified metabolites were classified into three categories according to the metabolic positions of a piperazine ring, imidazole ring, and N-acetyl moiety. The metabolic characteristics of KCZ in human were comparable to those in mouse. Moreover, three cyanide adducts of KCZ were identified in mouse and human liver microsomal incubates as "flags" to trigger additional toxicity study. The oxidation of piperazine into iminium ion is suggested as a biotransformation responsible for bioactivation. In summary, the metabolic characteristics of KCZ, including reactive metabolites, were comprehensively understood using a metabolomics approach.
P450 inhibitor ketoconazole increased the intratumor drug levels and antitumor activity of fenretinide in human neuroblastoma xenograft models.[Pubmed:28340497]
Int J Cancer. 2017 Jul 15;141(2):405-413.
We previously reported that concurrent Ketoconazole, an oral anti-fungal agent and P450 enzyme inhibitor, increased plasma levels of the cytotoxic retinoid, fenretinide (4-HPR) in mice. We have now determined the effects of concurrent Ketoconazole on 4-HPR cytotoxic dose-response in four neuroblastoma (NB) cell lines in vitro and on 4-HPR activity against two cell line-derived, subcutaneous NB xenografts (CDX) and three patient-derived NB xenografts (PDX). Cytotoxicity in vitro was assessed by DIMSCAN assay. Xenografted animals were treated with 4-HPR/LXS (240 mg/kg/day) + Ketoconazole (38 mg/kg/day) in divided oral doses in cycles of five continuous days a week. In one model, intratumoral levels of 4-HPR and metabolites were assessed by HPLC assay, and in two models intratumoral apoptosis was assessed by TUNEL assay, on Day 5 of the first cycle. Antitumor activity was assessed by Kaplan-Meier event-free survival (EFS). The in vitro cytotoxicity of 4-HPR was not affected by Ketoconazole (p >/= 0.06). Ketoconazole increased intratumoral levels of 4-HPR (p = 0.02), of the active 4-oxo-4-HPR metabolite (p = 0.04), and intratumoral apoptosis (p Ketoconazole increased EFS in both CDX models compared to 4-HPR/LXS-alone (p Ketoconazole also increased EFS in PDX models compared to controls (p Ketoconazole decreased 4-HPR metabolism with resultant increases of plasma and intratumoral drug levels and antitumor effects in neuroblastoma murine xenografts. These results support the clinical testing of concurrent Ketoconazole and oral fenretinide in neuroblastoma.
Ketoconazole inhibition of the bifunctional cytochrome P450c17 does not affect androgen formation from the endogenous lyase substrate. The catalytic site remains refractory in the course of intermediary hydroxyprogesterone processing.[Pubmed:1472102]
Biochem Pharmacol. 1992 Dec 15;44(12):2371-8.
The inhibition of the bifunctional steroidogenic cytochrome P450c17 (CYP17: steroid-17 alpha-hydroxylase/steroid-17,20-lyase) by the imidazole-type fungicide, [(+/-)-cis-1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl- methyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazine) (Ketoconazole), was investigated with the aim of differentiating between effects on androgen formation from exogenously added and endogenously produced 17 alpha-hydroxyprogesterone. Using microsomal membranes from rat testis, turnover of progesterone by P450c17 was competitively inhibited by Ketoconazole with KI = 0.40 microM. Ketoconazole did not affect the linear relationship between the ratio of productive events (corresponding to androgen formation rates) versus abortive events (corresponding to 17 alpha-hydroxyprogesterone formation rates) and the sum of catalytic events. This was an indication that this inhibitor did not interfere with intermediate processing by P450c17. Androgen formation from exogenous but not from endogenous 17 alpha-hydroxyprogesterone was competitively inhibited by Ketoconazole. The simultaneous conversion of 1 microM each of [3H]progesterone and 17 alpha-hydroxy[14C]progesterone was also reduced by Ketoconazole. Calculation of 3H/14C ratios in the 17 alpha-hydroxyprogesterone and androgen fractions revealed that the endogenous 17 alpha-hydroxyprogesterone pool was metabolized to androgens at rates 6.4, 11.6, 17.6 and 21.2-fold faster than the exogenous pool in the presence of 0.5, 1, 2 and 4 microM Ketoconazole, respectively; this value was only 4.0 in controls. It is concluded that Ketoconazole inhibits turnover of steroid ligands only when they approach the P450c17 active site in a substrate-state and that inhibition of androgen formation from progesterone is due to inhibition of the first catalytic step only. A model is described in which the P450c17 active site is refractory towards Ketoconazole when the intermediary steroid is retained and being processed at that site.
Inhibition of human adrenal steroidogenic enzymes in vitro by imidazole drugs including ketoconazole.[Pubmed:2724954]
J Steroid Biochem. 1989 Apr;32(4):515-24.
The effect of several imidazole containing drugs including keto on human adrenal 17 alpha-hydroxylase, 17,20-lyase, 21-hydroxylase, 11 beta-hydroxylase and 3 beta-hydroxysteroid dehydrogenase-isomerase (3 beta-HSD-I) activities was studied in vitro. The order of decreasing inhibitory potency as determined from ID50 values for both 17 alpha-hydroxylase (ID50 values ranged from 1.13-4.17 mumol/l) and 17,20-lyase (0.57-1.95 mumol/l) activities was: bifon greater than clot greater than keto greater than micon greater than econ greater than isocon greater than tiocon. Using [3H]progesterone (5.50-12.25 mumol/l) as the substrate for the 21-hydroxylase activity the order of decreasing inhibitory potency was: clot greater than bifon greater than isocon greater than micon greater than tiocon greater than econ greater than tiocon greater than keto. For the 11 beta-hydroxylation of [3H]deoxycortisol (1.48-2.34 mumol/l) the order of decreasing inhibitory potency was keto greater than bifon greater than clot greater than micon greater than econ greater than isocon greater than tiocon. The cytochrome P-450 dependent enzyme most sensitive to inhibition was 17,20-lyase and the least sensitive was 21-hydroxylase whereas the imidazole drugs were without effect on the cytochrome P-450 independent 3 beta-HSD-I activity. In agreement with previous results a common structural feature of the imidazole drugs having an inhibitory effect was the presence of aromatic rings on the N-1 substituent of the imidazole ring.


