Sitagliptin phosphate monohydratePotent DPP-4 inhibitor CAS# 654671-77-9 |
- PK 44 phosphate
Catalog No.:BCC2366
CAS No.:1017682-65-3
- Vildagliptin (LAF-237)
Catalog No.:BCC2112
CAS No.:274901-16-5
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
- Alogliptin Benzoate
Catalog No.:BCC1341
CAS No.:850649-62-6
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- DPPI 1c hydrochloride
Catalog No.:BCC2363
CAS No.:866396-34-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 654671-77-9 | SDF | Download SDF |
| PubChem ID | 11591741 | Appearance | Powder |
| Formula | C16H20F6N5O6P | M.Wt | 523.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MK-0431 phosphate monohydrate | ||
| Solubility | H2O : ≥ 33 mg/mL (63.06 mM) *"≥" means soluble, but saturation unknown. | ||
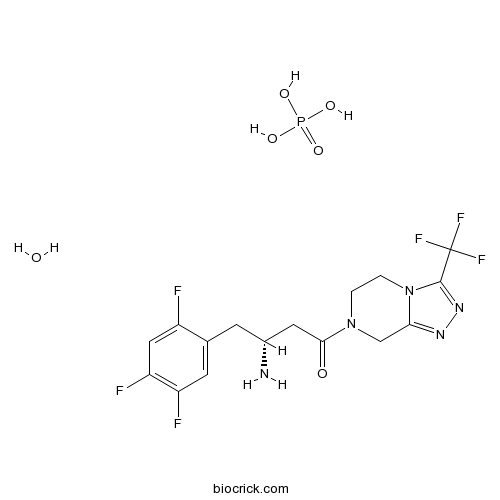
| Chemical Name | (3R)-3-amino-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one;phosphoric acid;hydrate | ||
| SMILES | C1CN2C(=NN=C2C(F)(F)F)CN1C(=O)CC(CC3=CC(=C(C=C3F)F)F)N.O.OP(=O)(O)O | ||
| Standard InChIKey | GQPYTJVDPQTBQC-KLQYNRQASA-N | ||
| Standard InChI | InChI=1S/C16H15F6N5O.H3O4P.H2O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22;1-5(2,3)4;/h4,6,9H,1-3,5,7,23H2;(H3,1,2,3,4);1H2/t9-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sitagliptin phosphate is a potent inhibitor of DPP-IV with IC50 of 19 nM in Caco-2 cell extracts. | |||||
| Targets | DPP-4 | |||||
| IC50 | 19 nM | |||||
| Cell experiment [1]: | |
| Cell lines | Endothelial progenitor cells (EPCs) and bone marrow mesenchymalstem cells(MSC) |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 14 d; 25 μmol/L |
| Applications | To determine whether sitagliptin treatment participated in enhancing the differentiation of EPCs and MSCs and cells expressing its ligand, SDF-1α, adipose tissues were co-cultured with sitagliptin (25 μmol/L) in M199 culture medium for 14 d and examined by flow cytometric analysis. The results show that compared with the 7 d cell culture, the numbers of EPCs [CD31/Sca-1+(double-stained) and CXCR4+ (single-stained)] were remarkably higher at day 14 in both the non-sitagliptin-treated (Si-T) group and the Si-T group |
| Animal experiment [2]: | |
| Animal models | ApoE−/−mice with the C57BL/6 genetic background |
| Dosage form | 200 mg/kg/day; oral taken |
| Application | In ApoE−/−mice, the sitagliptin group showed fewer atherosclerotic plaques than in controls (7.64±1.98% [range 4.62–10.13%] vs 12.91±1.15% [range 11.55–14.37%], p<0.001). Compared with control mice, atherosclerotic plaque areas decreased respectively 1.92- and 2.74-fold in the aortic root and abdominal aorta of mice fed sitagliptin (p=0.011 and p=0.006). Our data show that sitagliptin can inhibit the formation of atherosclerotic areas in entire aorta, aortic root and abdominal aorta of ApoE−/− mice. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Chua S, Sheu J J, Chen Y L, et al. Sitagliptin therapy enhances the number of circulating angiogenic cells and angiogenesis—evaluations< i> in vitro and in the rat critical limb ischemia model[J]. Cytotherapy, 2013, 15(9): 1148-1163. [2] Zeng Y, Li C, Guan M, et al. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK-and MAPK-dependent mechanisms[J]. Cardiovascular diabetology, 2014, 13(1): 32. | |

Sitagliptin phosphate monohydrate Dilution Calculator

Sitagliptin phosphate monohydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9109 mL | 9.5547 mL | 19.1095 mL | 38.219 mL | 47.7737 mL |
| 5 mM | 0.3822 mL | 1.9109 mL | 3.8219 mL | 7.6438 mL | 9.5547 mL |
| 10 mM | 0.1911 mL | 0.9555 mL | 1.9109 mL | 3.8219 mL | 4.7774 mL |
| 50 mM | 0.0382 mL | 0.1911 mL | 0.3822 mL | 0.7644 mL | 0.9555 mL |
| 100 mM | 0.0191 mL | 0.0955 mL | 0.1911 mL | 0.3822 mL | 0.4777 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The reverse phase UPLC is a method to simultaneously measure Sitagliptin phosphate monohydrate and Metformin hydrochloride in pharmaceutical dosage forms with LOD and LOQ of 0.2 μg/mL and 0.7 μg/mL respectively for Sitagliptin phosphate monohydrate.
Abstract
A retrospective cohort study was performed to investigate the clinical outcomes of sitagliption compared with metformin.
Abstract
The combination of sitagliptin and pioglitazone has been assessed for safety and efficacy in patients with type 2 diabetes.
Abstract
Sitagliptin is a DPP-4 inhibitor that inhibits the cleavage of GLP-1 leading to improved blood glucose control in patients with type 2 diabetes. Although it initially increased the percentage of cells expressing high levels of CD26, sitagliptin failed to alter systemic immune function in healthy volunteers at the end of 28-day treatment.
Abstract
Sitagliptin was evaluated for efficacy and safety in renal transplant recipients.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sitagliptin phosphate monohydrate is the phosphate salt of its active component, sitagliptin, with one molecule of water. Sitagliptin is a potent inhibitor of dipeptidyl peptidase 4 (DPP-4), an enzyme catalyzing the cleavage of peptides with an N-terminal alanine or proline amino acid residue, that selectively inhibits DPP-4 with 50% inhibition concentration IC50 value of 18 nM and shows no affinity towards other DDP enzymes (such as DDP-8 and DDP-9). The inhibition of DPP4 by sitagliptin has been found to be mediated by increasing levels of two DPP-4 substrates, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP). Sitagliptin is currently being investigated in the treatment of type II diabetes.
Reference
Gallwitz B. Review of sitagliptin phosphate: a novel treatment for type 2 diabetes. Vasc Health Risk Manag. 2007;3(2):203-10.
- Fmoc-Asn(Trt)-ol
Catalog No.:BCC2585
CAS No.:654670-89-0
- Acantrifoic acid A
Catalog No.:BCN6488
CAS No.:654663-85-1
- Phebalosin
Catalog No.:BCN4198
CAS No.:6545-99-9
- 1-Methoxyphaseollidin
Catalog No.:BCN7185
CAS No.:65428-13-9
- DL-Dab.2HCl
Catalog No.:BCC2669
CAS No.:65427-54-5
- Boc-Asn(Xan)-OH
Catalog No.:BCC3361
CAS No.:65420-40-8
- H-D-Asp-Ome
Catalog No.:BCC2896
CAS No.:65414-78-0
- Altholactone
Catalog No.:BCN4786
CAS No.:65408-91-5
- Morin hydrate
Catalog No.:BCC8214
CAS No.:654055-01-3
- Bestatin hydrochloride
Catalog No.:BCC3908
CAS No.:65391-42-6
- alpha-Isowighteone
Catalog No.:BCN4197
CAS No.:65388-03-6
- D-Chicoric Acid
Catalog No.:BCC8148
CAS No.:52248-48-3
- Sitagliptin phosphate
Catalog No.:BCC9148
CAS No.:654671-78-0
- (Z)-23-Coumaroylhederagenin
Catalog No.:BCN3748
CAS No.:654678-61-2
- Naftifine HCl
Catalog No.:BCC4806
CAS No.:65473-14-5
- Esculentoside A
Catalog No.:BCN5010
CAS No.:65497-07-6
- Cyclo(Pro-Ala)
Catalog No.:BCN2427
CAS No.:65556-33-4
- Europine N-oxide
Catalog No.:BCN1977
CAS No.:65582-53-8
- 1-Hydroxyacridone
Catalog No.:BCN7524
CAS No.:65582-54-9
- 4'-Demethylepipodophyllotoxin
Catalog No.:BCN5918
CAS No.:6559-91-7
- Cerbinal
Catalog No.:BCN4199
CAS No.:65597-42-4
- Cerberic acid
Catalog No.:BCN4200
CAS No.:65597-44-6
- Ophiopogonin D'
Catalog No.:BCN2645
CAS No.:65604-80-0
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
Simultaneous Determination of Sitagliptin Phosphate Monohydrate and Metformin Hydrochloride in Tablets by a Validated UPLC Method.[Pubmed:22396910]
Sci Pharm. 2012 Jan-Mar;80(1):139-52.
A novel approach was used to develop and validate a rapid, specific, accurate and precise reverse phase ultra performance liquid chromatographic (UPLC) method for the simultaneous determination of Sitagliptin phosphate monohydrate and Metformin hydrochloride in pharmaceutical dosage forms. The chromatographic separation was achieved on Aquity UPLC BEH C8 100 x 2.1 mm, 1.7 mum, column using a buffer consisting of 10 mM potassium dihydrogen phosphate and 2 mM hexane-1-sulfonic acid sodium salt (pH adjusted to 5.50 with diluted phosphoric acid) and acetonitrile as organic solvent in a gradient program. The flow rate was 0.2 mL min(-1) and the detection wavelength was 210 nm. The limit of detection (LOD) for Sitagliptin phosphate monohydrate and Metformin hydrochloride was 0.2 and 0.06 mug mL(-1), respectively. The limit of quantification (LOQ) for Sitagliptin phosphate monohydrate and Metformin hydrochloride was 0.7 and 0.2 mug mL(-1), respectively. This method was validated with respect to linearity, accuracy, precision, specificity and robustness. The method was also found to be stability-indicating.


