PK 44 phosphateDPP-IV inhibitor CAS# 1017682-65-3 |
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- NVP DPP 728 dihydrochloride
Catalog No.:BCC2365
CAS No.:247016-69-9
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- DPPI 1c hydrochloride
Catalog No.:BCC2363
CAS No.:866396-34-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1017682-65-3 | SDF | Download SDF |
| PubChem ID | 46208486 | Appearance | Powder |
| Formula | C17H16F5N7O | M.Wt | 429.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
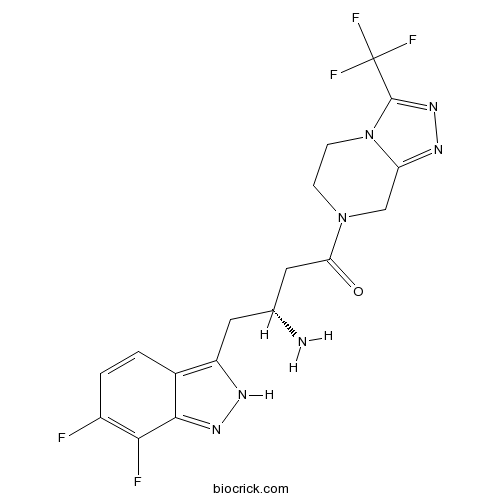
| Chemical Name | (3R)-3-amino-4-(6,7-difluoro-2H-indazol-3-yl)-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]butan-1-one | ||
| SMILES | C1CN2C(=NN=C2C(F)(F)F)CN1C(=O)CC(CC3=C4C=CC(=C(C4=NN3)F)F)N | ||
| Standard InChIKey | LIPWZZYFIVYJSI-MRVPVSSYSA-N | ||
| Standard InChI | InChI=1S/C17H16F5N7O/c18-10-2-1-9-11(24-26-15(9)14(10)19)5-8(23)6-13(30)28-3-4-29-12(7-28)25-27-16(29)17(20,21)22/h1-2,8H,3-7,23H2,(H,24,26)/t8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PK 44 phosphate is a potent inhibitor of dipeptidyl peptidase IV (DPP-IV) with an IC50 value of 15.8 nM. | |||||
| Targets | DPP-IV | |||||
| IC50 | 15.8 nM | |||||

PK 44 phosphate Dilution Calculator

PK 44 phosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3294 mL | 11.6469 mL | 23.2937 mL | 46.5875 mL | 58.2343 mL |
| 5 mM | 0.4659 mL | 2.3294 mL | 4.6587 mL | 9.3175 mL | 11.6469 mL |
| 10 mM | 0.2329 mL | 1.1647 mL | 2.3294 mL | 4.6587 mL | 5.8234 mL |
| 50 mM | 0.0466 mL | 0.2329 mL | 0.4659 mL | 0.9317 mL | 1.1647 mL |
| 100 mM | 0.0233 mL | 0.1165 mL | 0.2329 mL | 0.4659 mL | 0.5823 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PK 44 phosphate
Description:IC50: 15.8 nm (DPP-IV)
The protein encoded by the DPP4 gene is an antigenic enzyme expressed on the surface of most cell types and is associated with immune regulation, signal transduction and apoptosis. Inhibitors of dipeptidyl peptidase 4, also DPP-4 inhibitors or gliptins, are a class of oral hypoglycemics that block DPP-4. They can be used to treat diabetes mellitus type 2 (http://en.wikipedia.org/wiki/Dipeptidyl_peptidase-4_inhibitors). PK 44 phosphate is a potent inhibitor of dipeptidyl peptidase IV (DPP-IV).
In vitro: With a potent inhibitor of dipeptidyl peptidase IV (DPP-IV), PK 44 phosphate showed more than 1000-fold selectivity for DPP-IV over DPP-8 and DPP-9 [1].
In vivo: PK 44 phosphate was found to be able to improve glucose tolerance in a mouse oral glucose tolerance assay [1].
Clinical trial: PK 44 phosphate is currently in the preclinical development and no clinical trial is ongoing.
Reference:
[1] Tozer et al (2010) Indole- and indazole-based inhibitors of dipeptidyl peptidase IV for the treatment of type 2 diabetes. 32nd Annual National Medicinal Chemistry Symposium Poster 52.
- LPA2 antagonist 1
Catalog No.:BCC5438
CAS No.:1017606-66-4
- Ladanein
Catalog No.:BCN6670
CAS No.:10176-71-3
- Nevadensin
Catalog No.:BCN6806
CAS No.:10176-66-6
- Jaceidin
Catalog No.:BCN5832
CAS No.:10173-01-0
- Sanggenofuran B
Catalog No.:BCN7194
CAS No.:1017277-40-5
- 6alpha,16,18-Trihydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN1640
CAS No.:1017233-48-5
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
- Myzodendrone
Catalog No.:BCN7257
CAS No.:101705-37-7
- 6-O-p-Hydroxybenzoylaucubin
Catalog No.:BCN5831
CAS No.:1016987-87-3
- Sulfocostunolide A
Catalog No.:BCN5830
CAS No.:1016983-51-9
- Olivil monoacetate
Catalog No.:BCN4738
CAS No.:1016974-78-9
- Barpisoflavone A
Catalog No.:BCN4739
CAS No.:101691-27-4
- Boc-N-Me-Ser-OH
Catalog No.:BCC2613
CAS No.:101772-29-6
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- 7-O-Demethyl-3-isomangostin hydrate
Catalog No.:BCN7882
CAS No.:
- Desacetylmatricarin
Catalog No.:BCN7258
CAS No.:10180-88-8
- TG 100801 Hydrochloride
Catalog No.:BCC1997
CAS No.:1018069-81-2
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
Activity of Tedizolid in Methicillin-Resistant Staphylococcus aureus Experimental Foreign Body-Associated Osteomyelitis.[Pubmed:27550347]
Antimicrob Agents Chemother. 2016 Oct 21;60(11):6568-6572.
We compared tedizolid alone and tedizolid with rifampin to rifampin and vancomycin plus rifampin in a rat model of methicillin-resistant Staphylococcus aureus (MRSA) foreign body-associated osteomyelitis. The study strain was a prosthetic joint infection-associated isolate. Steady-state pharmacokinetics for intraperitoneal administration of tedizolid, vancomycin, and rifampin were determined in uninfected rats. MRSA was inoculated into the proximal tibia, and a wire was implanted. Four weeks later, the rats were treated intraperitoneally for 21 days with tedizolid (n = 14), tedizolid plus rifampin (n = 11), rifampin (n = 16), or vancomycin plus rifampin (n = 13). Seventeen rats received no treatment. After treatment, quantitative bone cultures were performed. Blood was obtained for determination of drug trough concentrations in the tedizolid and tedizolid plus rifampin groups. The mean peak plasma concentration and mean area under the concentration-time curve from time zero to 24 h for tedizolid were 12 mug/ml and 60 mug . h/ml, respectively. The bacterial loads in all treatment groups were significantly lower than those in the control group; those in the tedizolid- plus rifampin-treated animals were not significantly different from those in the vancomycin- plus rifampin-treated animals. The range of mean plasma trough concentrations in the tedizolid group was 0.44 to 0.73 mug/ml. Although neither tedizolid nor vancomycin resistance was detected in isolates recovered from bones, rifampin resistance was detected in 10 animals (63%) in the rifampin group, 8 animals (73%) in the tedizolid plus rifampin group, and a single animal (8%) in the vancomycin plus rifampin group. Tedizolid alone or tedizolid combined with rifampin was active in a rat model of MRSA foreign body-associated osteomyelitis. The emergence of rifampin resistance was noted in animals receiving tedizolid plus rifampin.
Zomepirac Acyl Glucuronide Is Responsible for Zomepirac-Induced Acute Kidney Injury in Mice.[Pubmed:27112166]
Drug Metab Dispos. 2016 Jul;44(7):888-96.
Glucuronidation, an important phase II metabolic route, is generally considered to be a detoxification pathway. However, acyl glucuronides (AGs) have been implicated in the toxicity of carboxylic acid drugs due to their electrophilic reactivity. Zomepirac (ZP) was withdrawn from the market because of adverse effects such as renal toxicity. Although ZP is mainly metabolized to acyl glucuronide (ZP-AG) by UDP-glucuronosyltransferase, the role of ZP-AG in renal toxicity is unknown. In this study, we established a ZP-induced kidney injury mouse model by pretreatment with tri-o-tolyl phosphate (TOTP), a nonselective esterase inhibitor, and l-buthionine-(S,R)-sulfoximine (BSO), a glutathione synthesis inhibitor. The role of ZP-AG in renal toxicity was investigated using this model. The model showed significant increases in blood urea nitrogen (BUN) and creatinine (CRE), but not alanine aminotransferase. The ZP-AG concentrations were elevated by cotreatment with TOTP in the plasma and liver and especially in the kidney. The ZP-AG concentrations in the kidney correlated with values for BUN and CRE. Upon histopathological examination, vacuoles and infiltration of mononuclear cells were observed in the model mouse. In addition to immune-related responses, oxidative stress markers, such as the glutathione/disulfide glutathione ratio and malondialdehyde levels, were different in the mouse model. The suppression of ZP-induced kidney injury by tempol, an antioxidant agent, suggested the involvement of oxidative stress in ZP-induced kidney injury. This is the first study to demonstrate that AG accumulation in the kidney by TOTP and BSO treatment could explain renal toxicity and to show the in vivo toxicological potential of AGs.
Thymidine esters as substrate analogue inhibitors of angiogenic enzyme thymidine phosphorylase in vitro.[Pubmed:27955923]
Bioorg Chem. 2017 Feb;70:44-56.
Thymidine phosphorylase (TP) catalyzes the cleavage of thymidine into thymine and 2-deoxy-alpha-d-ribose-1-phosphate. Elevated activity of TP prevents apoptosis, and induces angiogenesis which ultimately leads to tumor growth and metastasis. Critical role of TP in cancer progression makes it a valid target in anti-cancer research. Discovery of small molecules as TP inhibitors is vigorously pursued in cancer therapy. In the present study, we functionalized thymidine as benzoyl ester to synthesize compounds 3-16. In vitro evaluation of thymidine esters for their thymidine phosphorylase inhibition activity was subsequently carried out. Compounds 4, 10, 14, and 15 showed good activities with lower IC50 values than the standard, 7-deazaxanthine (IC50=41.0+/-1.63muM). Among them, compound 14 showed five folds higher activity (IC50=7.5+/-0.8muM), while 4 (IC50=18.5+/-1.0muM) and 10 (IC50=18.8+/-1.2muM) showed two folds higher activity than the standard. Compound 15 showed slightly better activity (IC50=33.3+/-1.5muM) to the standard. Potent compounds were further subjected to kinetic and molecular docking studies to identify their mode of inhibition, and to study their interactions with the protein at atomic level, respectively. All active compounds were non-cytotoxic to mouse fibroblast 3T3 cell line. These results identify thymidine esters as substrate analogue (substrate-like) inhibitors of angiogenic enzyme thymidine phosphorylase for further studies.
Model Linking Plasma and Intracellular Tenofovir/Emtricitabine with Deoxynucleoside Triphosphates.[Pubmed:27832147]
PLoS One. 2016 Nov 10;11(11):e0165505.
The coformulation of the nucleos(t)ide analogs (NA) tenofovir (TFV) disoproxil fumarate (TDF) and emtricitabine (FTC) is approved for HIV-infection treatment and prevention. Plasma TFV and FTC undergo complicated hybrid processes to form, accumulate, and retain as their active intracellular anabolites: TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP). Such complexities manifest in nonlinear intracellular pharmacokinetics (PK). In target cells, TFV-DP/FTC-TP compete with endogenous deoxynucleoside triphosphates (dNTP) at the active site of HIV reverse transcriptase, underscoring the importance of analog:dNTP ratios for antiviral efficacy. However, NA such as TFV and FTC have the potential to disturb the dNTP pool, which could augment or reduce their efficacies. We conducted a pharmacokinetics-pharmacodynamics (PKPD) study among forty subjects receiving daily TDF/FTC (300 mg/200 mg) from the first-dose to pharmacological intracellular steady-state (30 days). TFV/FTC in plasma, TFV-DP/FTC-TP and dNTPs in peripheral blood mononuclear cells (PBMC) were quantified using validated LC/MS/MS methodologies. Concentration-time data were analyzed using nonlinear mixed effects modeling (NONMEM). Formations and the accumulation of intracellular TFV-DP/FTC-TP was driven by plasma TFV/FTC, which was described by a hybrid of first-order formation and saturation. An indirect response link model described the interplay between TFV-DP/FTC-TP and the dNTP pool change. The EC50 (interindividual variability, (%CV)) of TFV-DP and FTC-TP on the inhibition of deoxyadenosine triphosphate (dATP) and deoxycytidine triphosphate (dCTP) production were 1020 fmol/106 cells (130%) and 44.4 pmol/106 cells (82.5%), resulting in (90% prediction interval) 11% (0.45%, 53%) and 14% (2.6%, 35%) reductions. Model simulations of analog:dNTP molar ratios using IPERGAY dosing suggested that FTC significantly contributes to the protective effect of preexposure prophylaxis (PrEP). Simulation-based intracellular operational multiple dosing half-lives of TFV-DP and FTC-TP were 6.7 days and 33 hours. This model described the formation of intracellular TFV-DP/FTC-TP and the interaction with dNTPs, and can be used to simulate analog:dNTP time course for various dosing strategies.
Construction and evaluation in vitro and in vivo of tedizolid phosphate loaded cationic liposomes.[Pubmed:28920493]
J Liposome Res. 2018 Dec;28(4):322-330.
First, the SA-TDZA-Lips were prepared by reverse-phase evaporation method. Then, the drug release behaviour was evaluated by dynamic membrane dialysis in vitro and the preliminary safety was evaluated by haemolysis method. Finally, with tedizolid phosphate injection (TDZA-Inj) and tedizolid phosphate loaded liposomes (TDZA-Lips) as the control groups, the pharmacokinetic characteristic and tissues distribution of SA-TDZA-Lips were evaluated after intravenous injection. As a result, the stearylamine modified tedizolid phosphate liposomal delivery system was constructed successfully and the particle size was 194.9 +/- 2.93 nm. The encapsulation efficiency (EE) was 53.52 +/- 2.18%. The in vitro release of SA-TDZA-Lips was in accordance with Weibull equation. And there was no haemolysis happened, which indicated good preliminary safety for injection. The results of pharmacokinetics showed that the t1/2beta increased by 0.74 times and 0.51 times higher than that of TDZA-Inj group and TDZA-Lips group, respectively. The MRT of SA-TDZA-Lips was 1.30 and 1.09 times higher than that of TDZA-Inj group and TDZA-Lips group, respectively. The AUC was 2.40 times and 0.23 times higher than that of TDZA-Inj group and TDZA-Lips group, respectively. The tissue distribution results showed that the relative uptake rate (Re) of TDZA in the lung was 1.527, which indicated the targeting. In conclusion, the SA-TDZA-Lips prepared in this study had several advantages like positive charge, strong cell affinity, prolonged circulation time in vivo, sustained release effect, and increased drug concentration in lungs. All advantages above provided significant clinical value of application for the treatment of bacterial pneumonia with tedizolid phosphate.


