LadaneinCAS# 10176-71-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10176-71-3 | SDF | Download SDF |
| PubChem ID | 3084066 | Appearance | Yellow powder |
| Formula | C17H14O6 | M.Wt | 314.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
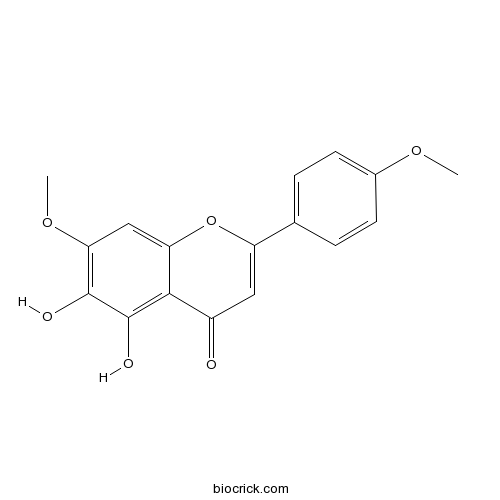
| Chemical Name | 5,6-dihydroxy-7-methoxy-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=CC(=O)C3=C(C(=C(C=C3O2)OC)O)O | ||
| Standard InChIKey | UUQJTIHOVGMQIH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O6/c1-21-10-5-3-9(4-6-10)12-7-11(18)15-13(23-12)8-14(22-2)16(19)17(15)20/h3-8,19-20H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ladanein displays moderate (20-40 microM) activities against K562, K562R (imatinib-resistant), and 697 human leukemia cell lines . 2. Ladanein possesses free radicals DPPH and ABTS +.scavenging activity. 3. Ladanein is a phytochemicals inhibitor that is known to disrupt the interactions of core and other hepatitis C virus (HCV) proteins. |
| Targets | HCV |

Ladanein Dilution Calculator

Ladanein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1817 mL | 15.9084 mL | 31.8167 mL | 63.6335 mL | 79.5418 mL |
| 5 mM | 0.6363 mL | 3.1817 mL | 6.3633 mL | 12.7267 mL | 15.9084 mL |
| 10 mM | 0.3182 mL | 1.5908 mL | 3.1817 mL | 6.3633 mL | 7.9542 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6363 mL | 1.2727 mL | 1.5908 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6363 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nevadensin
Catalog No.:BCN6806
CAS No.:10176-66-6
- Jaceidin
Catalog No.:BCN5832
CAS No.:10173-01-0
- Sanggenofuran B
Catalog No.:BCN7194
CAS No.:1017277-40-5
- 6alpha,16,18-Trihydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN1640
CAS No.:1017233-48-5
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
- Myzodendrone
Catalog No.:BCN7257
CAS No.:101705-37-7
- 6-O-p-Hydroxybenzoylaucubin
Catalog No.:BCN5831
CAS No.:1016987-87-3
- Sulfocostunolide A
Catalog No.:BCN5830
CAS No.:1016983-51-9
- Olivil monoacetate
Catalog No.:BCN4738
CAS No.:1016974-78-9
- Barpisoflavone A
Catalog No.:BCN4739
CAS No.:101691-27-4
- Cyclo(Phe-Hpro)
Catalog No.:BCN2425
CAS No.:1016899-94-7
- Cyclo(Hpro-Leu)
Catalog No.:BCN2430
CAS No.:1016899-93-6
- LPA2 antagonist 1
Catalog No.:BCC5438
CAS No.:1017606-66-4
- PK 44 phosphate
Catalog No.:BCC2366
CAS No.:1017682-65-3
- Boc-N-Me-Ser-OH
Catalog No.:BCC2613
CAS No.:101772-29-6
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- 7-O-Demethyl-3-isomangostin hydrate
Catalog No.:BCN7882
CAS No.:
- Desacetylmatricarin
Catalog No.:BCN7258
CAS No.:10180-88-8
- TG 100801 Hydrochloride
Catalog No.:BCC1997
CAS No.:1018069-81-2
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
Activity of ladanein on leukemia cell lines and its occurrence in Marrubium vulgare.[Pubmed:19644796]
Planta Med. 2010 Jan;76(1):86-7.
Three methoxylated flavones isolated from Marrubium peregrinum - Ladanein, scutellarein-5,7,4'-trimethyl ether, and scutellarein-5,6,7,4'-tetramethyl ether - were assayed for their cytotoxicity towards a recently developed dasatinib-resistant murine leukemia cell line (DA1-3b/M2 (BCR-ABL)), together with the structurally related non-methylated flavone scutellarein. The most active compound, Ladanein, was looked for in 20 common Lamiaceae species by a quick HPLC screening. Among the possible positive results, the most interesting source was found to be Marrubium vulgare, which led to the isolation and identification of Ladanein for the first time in this species. Ladanein also displayed moderate (20-40 microM) activities against K562, K562R (imatinib-resistant), and 697 human leukemia cell lines but was toxic neither to MOLM13 nor to human peripheral blood mononuclear cells. This work provides a common natural source for the hemi-synthesis of future Ladanein-derived flavones and the study of their antileukemic activity.
In silico studies of medicinal compounds against hepatitis C capsid protein from north India.[Pubmed:25002815]
Bioinform Biol Insights. 2014 Jun 23;8:159-68.
Hepatitis viral infection is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). Over one million people are estimated to be persistently infected with hepatitis C virus (HCV) worldwide. As capsid core protein is the key element in spreading HCV; hence, it is considered to be the superlative target of antiviral compounds. Novel drug inhibitors of HCV are in need to complement or replace the current treatments such as pegylated interferon's and ribavirin as they are partially booming and beset with various side effects. Our study was conducted to predict 3D structure of capsid core protein of HCV from northern part of India. Core, the capsid protein of HCV, handles the assembly and packaging of HCV RNA genome and is the least variable of all the ten HCV proteins among the six HCV genotypes. Therefore, we screened four phytochemicals inhibitors that are known to disrupt the interactions of core and other HCV proteins such as (a) epigallocatechin gallate (EGCG), (b) Ladanein, (c) naringenin, and (d) silybin extracted from medicinal plants; targeted against active site of residues of HCV-genotype 3 (G3) (Q68867) and its subtypes 3b (Q68861) and 3g (Q68865) from north India. To study the inhibitory activity of the recruited flavonoids, we conducted a quantitative structure-activity relationship (QSAR). Furthermore, docking interaction suggests that EGCG showed a maximum number of hydrogen bond (H-bond) interactions with all the three modeled capsid proteins with high interaction energy followed by naringenin and silybin. Thus, our results strongly correlate the inhibitory activity of the selected bioflavonoid. Finally, the dynamic predicted capsid protein molecule of HCV virion provides a general avenue to target structure-based antiviral compounds that support the hypothesis that the screened inhibitors for viral capsid might constitute new class of potent agents but further confirmation is necessary using in vitro and in vivo studies.


