DesacetylmatricarinCAS# 10180-88-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10180-88-8 | SDF | Download SDF |
| PubChem ID | 99114 | Appearance | Powder |
| Formula | C15H18O4 | M.Wt | 262.30 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
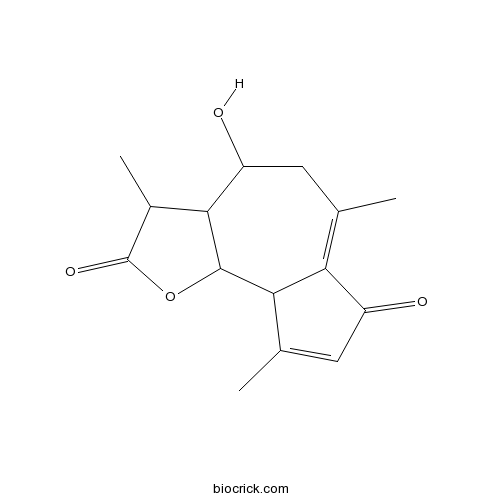
| Chemical Name | 4-hydroxy-3,6,9-trimethyl-3,3a,4,5,9a,9b-hexahydroazuleno[4,5-b]furan-2,7-dione | ||
| SMILES | CC1C2C(CC(=C3C(C2OC1=O)C(=CC3=O)C)C)O | ||
| Standard InChIKey | YMUOZXZDDBRJEP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H18O4/c1-6-4-10(17)13-8(3)15(18)19-14(13)12-7(2)5-9(16)11(6)12/h5,8,10,12-14,17H,4H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Desacetylmatricarin has anti-allergic property, it shows a potent inhibitory activity upon the beta-hexosaminidase release from RBL-2H3 cells in a dose-dependent manner and the IC50 of 7.5 microM. |

Desacetylmatricarin Dilution Calculator

Desacetylmatricarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8124 mL | 19.0621 mL | 38.1243 mL | 76.2486 mL | 95.3107 mL |
| 5 mM | 0.7625 mL | 3.8124 mL | 7.6249 mL | 15.2497 mL | 19.0621 mL |
| 10 mM | 0.3812 mL | 1.9062 mL | 3.8124 mL | 7.6249 mL | 9.5311 mL |
| 50 mM | 0.0762 mL | 0.3812 mL | 0.7625 mL | 1.525 mL | 1.9062 mL |
| 100 mM | 0.0381 mL | 0.1906 mL | 0.3812 mL | 0.7625 mL | 0.9531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-O-Demethyl-3-isomangostin hydrate
Catalog No.:BCN7882
CAS No.:
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- Boc-N-Me-Ser-OH
Catalog No.:BCC2613
CAS No.:101772-29-6
- PK 44 phosphate
Catalog No.:BCC2366
CAS No.:1017682-65-3
- LPA2 antagonist 1
Catalog No.:BCC5438
CAS No.:1017606-66-4
- Ladanein
Catalog No.:BCN6670
CAS No.:10176-71-3
- Nevadensin
Catalog No.:BCN6806
CAS No.:10176-66-6
- Jaceidin
Catalog No.:BCN5832
CAS No.:10173-01-0
- Sanggenofuran B
Catalog No.:BCN7194
CAS No.:1017277-40-5
- 6alpha,16,18-Trihydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN1640
CAS No.:1017233-48-5
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
- Myzodendrone
Catalog No.:BCN7257
CAS No.:101705-37-7
- TG 100801 Hydrochloride
Catalog No.:BCC1997
CAS No.:1018069-81-2
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
TLC and HPLC characteristics of desacetylmatricarin, leucodin, achillin and their 8alpha-angeloxy-derivatives.[Pubmed:12889533]
Pharmazie. 2003 Jul;58(7):487-90.
Five guaianolides, including two pairs of isomers, from a Hungarian taxon of the Achillea millefolium group were characterized analytically. Different chromatographic systems on TLC and HPLC were developed for the analysis of these compounds. TLC of leucodin, 8alpha-angeloxy-leucodin, achillin, 8alpha-angeloxy-achillin and Desacetylmatricarin was performed on silica gel using dichloromethaneacetone and cyclohexane-ethylacetate mixtures as mobile phases. HPLC on stationary phases as LiChrospher RP2, LiChrospher RP8, LiChrospher RP18e, Hypersil BDS C18 and Aquasil C18 required isocratic and gradient systems with different methanol-water mixtures as mobile phases. The presented RF values and retention times allow the identification of the respective 2-oxo-guaianolides which are marker substances for certain non-proazulene containing species. Their TLC and HPLC fingerprints are compared to those of proazulene containing species and are relevant for quality control.
Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines.[Pubmed:19107850]
Phytother Res. 2009 May;23(5):672-6.
The antiproliferative activities of n-hexane, chloroform, aqueous-methanol and aqueous extracts of the aerial parts of the Achillea millefolium aggregate on three human tumour cell lines were investigated by means of MTT assays. The chloroform-soluble extract exerted high tumour cell proliferation inhibitory activities on HeLa and MCF-7 cells, and a moderate effect on A431 cells; accordingly, it was subjected to detailed bioactivity-guided fractionation. As a result of the multistep chromatographic purifications (VLC, CPC, PLC, gel filtration), five flavonoids (apigenin, luteolin, centaureidin, casticin and artemetin) and five sesquiterpenoids (paulitin, isopaulitin, psilostachyin C, Desacetylmatricarin and sintenin) were isolated and identified by spectroscopic methods. The antiproliferative assay demonstrated that centaureidin is the most effective constituent of the aerial parts of yarrow: high cell growth inhibitory activities were observed especially on HeLa (IC(50) 0.0819 microm) and MCF-7 (IC(50) 0.1250 microm) cells. Casticin and paulitin were also highly effective against all three tumour cell lines (IC(50) 1.286-4.76 microm), while apigenin, luteolin and isopaulitin proved to be moderately active (IC(50) 6.95-32.88 microm). Artemetin, psilostachyin C, Desacetylmatricarin and sintenin did not display antiproliferative effects against these cell lines. This is the first report on the occurrence of seco-pseudoguaianolides (paulitin, isopaulitin and psilostachyin C) in the Achillea genus.
Sesquiterpenes and flavonoid aglycones from a Hungarian taxon of the Achillea millefolium group.[Pubmed:12562079]
Z Naturforsch C. 2002 Nov-Dec;57(11-12):976-82.
The investigation of a dichloromethane extract of flower heads of a Hungarian taxon of the Achillea millefolium group led to the isolation of three flavonoid aglycones, one triterpene, one germacranolide and five guaianolides. Their structures were elucidated by UV-VIS, EI- and CI-MS, 1H NMR and 13C NMR spectroscopic methods as well as by 2D-NMR studies and by selective 1D-NOE experiments. Besides apigenin, luteolin and centaureidin, beta-sitosterol, 3beta-hydroxy-11alpha,13-dihydro-costunolide, Desacetylmatricarin, leucodin, achillin, 8alpha-angeloxy-leucodin and 8alpha-angeloxy-achillin were isolated. Both latter substances are reported here for the first time. Their NMR data were compared with those of the other guaianolides. The stereochemistry of 3beta-hydroxy-11alpha,13-dihydro-costunolide was discussed and compared with data of the literature.
Desacetylmatricarin, an anti-allergic component from Taraxacum platycarpum.[Pubmed:9741305]
Planta Med. 1998 Aug;64(6):577-8.
The bioassay-guided fractionation of Taraxacum platycarpum (Compositae) extract led to the isolation of a Desacetylmatricarin (1) as an active principle responsible for the anti-allergic property. It showed a potent inhibitory activity upon the beta-hexosaminidase release from RBL-2H3 cells in a dose-dependent manner and the IC50 was 7.5 microM. Two structurally related guaianolide sesquiterpenes, achillin and leucodin, were also examined and their IC50 values were determined as 100 microM and 80 microM, respectively.


