7-O-Demethyl-3-isomangostin hydrateCAS# N/A |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | N/A | SDF | Download SDF |
| PubChem ID | 102004512 | Appearance | Yellow powder |
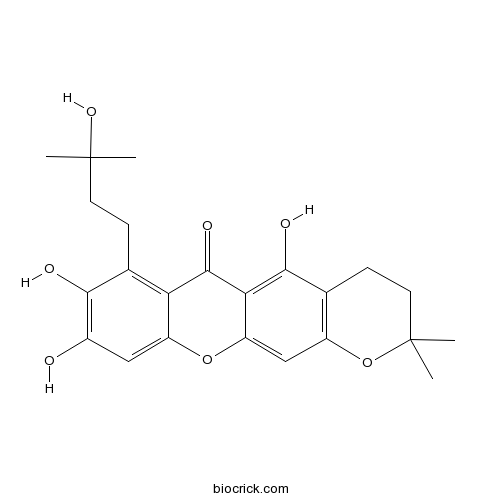
| Formula | C23H26O7 | M.Wt | 414.5 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Synonyms | 7-O-Demethyl-3-isomagosti hydrate | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,8,9-trihydroxy-7-(3-hydroxy-3-methylbutyl)-2,2-dimethyl-3,4-dihydropyrano[3,2-b]xanthen-6-one | ||
| SMILES | CC1(CCC2=C(O1)C=C3C(=C2O)C(=O)C4=C(C(=C(C=C4O3)O)O)CCC(C)(C)O)C | ||
| Standard InChIKey | ODPJZFJTDFGSOM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H26O7/c1-22(2,28)7-5-12-17-15(9-13(24)19(12)25)29-16-10-14-11(6-8-23(3,4)30-14)20(26)18(16)21(17)27/h9-10,24-26,28H,5-8H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | trans-Communic acid exhibits protective effects against UVB-induced skin aging by suppressing UVB-induced MMP-1 expression. trans-Communic acid shows considerable cytotoxicity against four human cancer cell lines in vitro.It also shows good activity against Mycobacterium aurum (MIC of 13.2 microM). |
| Targets | AP-1 | JNK | Akt | MAPK | PI3K | MMP(e.g.TIMP) | Antifection |
| In vitro | Brown Pine Leaf Extract and Its Active Component Trans-Communic Acid Inhibit UVB-Induced MMP-1 Expression by Targeting PI3K.[Pubmed: 26066652]PLoS One. 2015 Jun 11;10(6):e0128365.
Japanese red pine (Pinus densiflora) is widely present in China, Japan, and Korea. Its green pine leaves have traditionally been used as a food as well as a coloring agent. After being shed, pine leaves change their color from green to brown within two years, and although the brown pine leaves are abundantly available, their value has not been closely assessed. Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae).[Pubmed: 19755141]J Ethnopharmacol. 2009 Dec 10;126(3):500-5.The antimycobacterial activity of Juniperus communis was attributed to a sesquiterpene identified as longifolene (1) and two diterpenes, characterised as totarol (2) and trans-Communic acid (3). |
| Structure Identification | Arch Pharm Res. 2011 Dec;34(12):2043-9.A new lignan glycoside from Juniperus rigida.[Pubmed: 22210029 ]A new lignan glycoside, named juniperigiside (1) was isolated from the CHCl(3) soluble fraction of the MeOH extract of stems and leaves of Juniperus rigida S.et Z.
|

7-O-Demethyl-3-isomangostin hydrate Dilution Calculator

7-O-Demethyl-3-isomangostin hydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4125 mL | 12.0627 mL | 24.1255 mL | 48.2509 mL | 60.3136 mL |
| 5 mM | 0.4825 mL | 2.4125 mL | 4.8251 mL | 9.6502 mL | 12.0627 mL |
| 10 mM | 0.2413 mL | 1.2063 mL | 2.4125 mL | 4.8251 mL | 6.0314 mL |
| 50 mM | 0.0483 mL | 0.2413 mL | 0.4825 mL | 0.965 mL | 1.2063 mL |
| 100 mM | 0.0241 mL | 0.1206 mL | 0.2413 mL | 0.4825 mL | 0.6031 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- Boc-N-Me-Ser-OH
Catalog No.:BCC2613
CAS No.:101772-29-6
- PK 44 phosphate
Catalog No.:BCC2366
CAS No.:1017682-65-3
- LPA2 antagonist 1
Catalog No.:BCC5438
CAS No.:1017606-66-4
- Ladanein
Catalog No.:BCN6670
CAS No.:10176-71-3
- Nevadensin
Catalog No.:BCN6806
CAS No.:10176-66-6
- Jaceidin
Catalog No.:BCN5832
CAS No.:10173-01-0
- Sanggenofuran B
Catalog No.:BCN7194
CAS No.:1017277-40-5
- 6alpha,16,18-Trihydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN1640
CAS No.:1017233-48-5
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
- Myzodendrone
Catalog No.:BCN7257
CAS No.:101705-37-7
- 6-O-p-Hydroxybenzoylaucubin
Catalog No.:BCN5831
CAS No.:1016987-87-3
- Desacetylmatricarin
Catalog No.:BCN7258
CAS No.:10180-88-8
- TG 100801 Hydrochloride
Catalog No.:BCC1997
CAS No.:1018069-81-2
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9


