TG 100801 HydrochlorideVEGFr2 inhibitor CAS# 1018069-81-2 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1018069-81-2 | SDF | Download SDF |
| PubChem ID | 24823568 | Appearance | Powder |
| Formula | C33H31Cl2N5O3 | M.Wt | 616.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 5 mg/mL (8.11 mM; Need ultrasonic) | ||
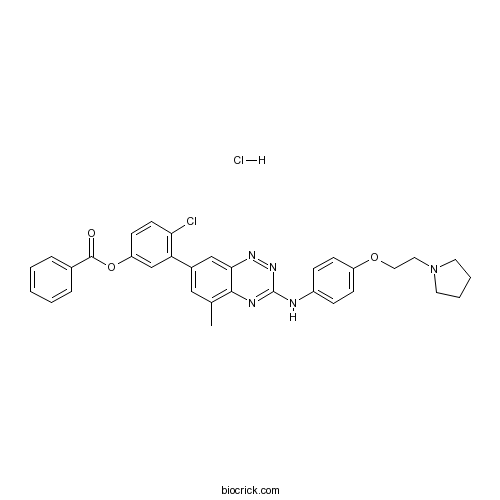
| Chemical Name | [4-chloro-3-[5-methyl-3-[4-(2-pyrrolidin-1-ylethoxy)anilino]-1,2,4-benzotriazin-7-yl]phenyl] benzoate;hydrochloride | ||
| SMILES | CC1=C2C(=CC(=C1)C3=C(C=CC(=C3)OC(=O)C4=CC=CC=C4)Cl)N=NC(=N2)NC5=CC=C(C=C5)OCCN6CCCC6.Cl | ||
| Standard InChIKey | VLWKPMDUDFMWBO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H30ClN5O3.ClH/c1-22-19-24(28-21-27(13-14-29(28)34)42-32(40)23-7-3-2-4-8-23)20-30-31(22)36-33(38-37-30)35-25-9-11-26(12-10-25)41-18-17-39-15-5-6-16-39;/h2-4,7-14,19-21H,5-6,15-18H2,1H3,(H,35,36,38);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TG 100801 Hydrochloride is a prodrug that generates TG 100572 by de-esterification in development to treat age-related macular degeneration. TG 100572 is a multi-targeted kinase inhibitor which inhibits receptor tyrosine kinases and Src kinases; has IC50s of 2, 7, 2, 16, 13, 5, 0.5, 6, 0.1, 0.4, 1, 0.2 for VEGFR1, VEGFR2, FGFR1, FGFR2, PDGFRβ, Fgr, Fyn, Hck, Lck, Lyn, Src, Yes, respectively.In Vitro:TG 100801 is a topically administered prodrug delivered as an eye drop that is readily converted to the active TG 100572 in the eye.TG 100572 is shown to inhibit hRMVEC cell proliferation, with an IC50 of 610±72 nM[1]. TG 100801 is formed by derivitization of a phenolic moiety in TG100572 to yield an ester. It displays excellent balance of stability (physical and chemical) with hydrolysis rate. On its own, TG 100801 does not display meaningful anti-kinase activity, as the ester group blocks key interactions with kinase active sites, however exposure to esterases (abundant in mammalian tissues) rapidly liberates active TG100572.TG 100572 shows sub-nanomolar activity against the Src family as well as RTK such as VEGFR1 and R2, FGFR1 and R2, and PDGFRβ. TG 100572 inhibits vascular endothelial cell proliferation (ED50=610±71 nM) and blocks VEGF-induced phosphorylation of extracellular signal-regulated kinase. TG 100572 induces apoptosis in rapidly proliferating, but not quiescent, endothelial cell cultures[2].In Vivo:TG 100801 exhibits excellent ocular pharmacokinetics and poor systemic circulation and shows good efficacy in the laser induced choroidal neovascularization model. A concentration of 23.4 µM (Cmax) of TG 100572 is reached in 30 min (Tmax)=0.5 h) in the choroid and the sclera. However, the levels of TG 100572 in the retina are relatively low. The half-life of TG 100572 in ocular tissues is very short; hence, the compound is administered topically minimum t.i.d. to maintain appropriate drug levels in the eye. The maximum concentration one can achieve in formulations using TG 100572 is 0.7% w/v[1]. TG 100801 nor TG100572 are detectable in plasma following topical delivery of TG 100801, and adverse safety signals (such as weight loss) are not observed even with prolonged dosing schedules. Topical TG 100801 significantly suppresses laser-induced choroidal neovascularlization in mice, and reduces fluorescein leakage from the vasculature and retinal thickening measured by optical coherence tomography in a rat model or retinal vein occlusion.Systemic delivery of TG 100572 in a murine model of laser-induced choroidal neovascularization (CNV) causes significant suppression of CNV, but with an associated weight loss suggestive of systemic toxicity[2]. References: | |||||

TG 100801 Hydrochloride Dilution Calculator

TG 100801 Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.622 mL | 8.1098 mL | 16.2195 mL | 32.4391 mL | 40.5489 mL |
| 5 mM | 0.3244 mL | 1.622 mL | 3.2439 mL | 6.4878 mL | 8.1098 mL |
| 10 mM | 0.1622 mL | 0.811 mL | 1.622 mL | 3.2439 mL | 4.0549 mL |
| 50 mM | 0.0324 mL | 0.1622 mL | 0.3244 mL | 0.6488 mL | 0.811 mL |
| 100 mM | 0.0162 mL | 0.0811 mL | 0.1622 mL | 0.3244 mL | 0.4055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TG 100801 is currently in a clinical trial as a first in class, VEGFr2 targeting, topically applied compound for the treatment of AMD. The formation of new blood vessels (angiogenesis), blood vessel leakage, and inflammation contribute to the progression of the eye disease, age-related macular degeneration (AMD), which is the leading cause of irreversible, severe loss of vision in people 55 years of age and older in the developed world. TG100801 is a new drug that inhibits ocular angiogenesis, vascular leak, and inflammation in laboratory studies, and may have great utility in the treatment of diseases such as AMD.
- Desacetylmatricarin
Catalog No.:BCN7258
CAS No.:10180-88-8
- 7-O-Demethyl-3-isomangostin hydrate
Catalog No.:BCN7882
CAS No.:
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- Boc-N-Me-Ser-OH
Catalog No.:BCC2613
CAS No.:101772-29-6
- PK 44 phosphate
Catalog No.:BCC2366
CAS No.:1017682-65-3
- LPA2 antagonist 1
Catalog No.:BCC5438
CAS No.:1017606-66-4
- Ladanein
Catalog No.:BCN6670
CAS No.:10176-71-3
- Nevadensin
Catalog No.:BCN6806
CAS No.:10176-66-6
- Jaceidin
Catalog No.:BCN5832
CAS No.:10173-01-0
- Sanggenofuran B
Catalog No.:BCN7194
CAS No.:1017277-40-5
- 6alpha,16,18-Trihydroxycleroda-3,13-dien-15,16-olide
Catalog No.:BCN1640
CAS No.:1017233-48-5
- (S)-(+)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8399
CAS No.:101711-78-8
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
- GW791343 dihydrochloride
Catalog No.:BCC1613
CAS No.:1019779-04-4
[TG-FTIR analysis of thermal degradation of bischofite's crystals with aniline hydrochloride].[Pubmed:21942016]
Guang Pu Xue Yu Guang Pu Fen Xi. 2011 Jul;31(7):1747-51.
The dehydration of bischofite is the key to the use of magnesium resource. Double salts or complex method is an important way to prepare anhydrous magnesium chloride. The crystals of bischofite and aniline hydrochloride were prepared through lowering the temperature of the mixture's aquatic solution slowly. The pyrolysis of aniline hydrochloride, bischofite and the crystals was qualitatively analyzed by TG-FTIR under 400 degrees C. We infered the reaction process through qualitative detection of escaping gas of each decomposition step. The Experimental results showed that there were three steps in the thermal decomposition of bischofite. In the first step, nearly four crystallized waters decomposed, while hydrolysis and decomposition took place together in the next two steps, but hydrolysis was the main reaction at the lower temperature (205-235 degrees C), and comparative decomposition was the main one at the higher temperature (235-287 degrees C). In the experimental temperature range, aniline hydrochloride didn't decompose. Water and aniline hydrochloride left the crystals at different temperature, and no hydrolysis reaction occurred. Anhydrous magnesium chloride can be prepared though this way.


