Cerberic acidCAS# 65597-44-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 65597-44-6 | SDF | Download SDF |
| PubChem ID | 71439415 | Appearance | Yellow powder |
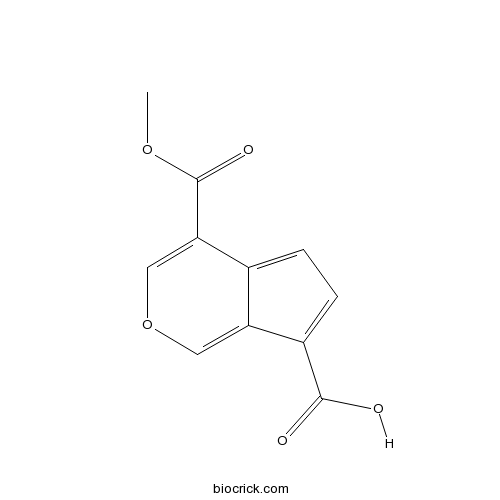
| Formula | C11H8O5 | M.Wt | 220.2 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-methoxycarbonylcyclopenta[c]pyran-7-carboxylic acid | ||
| SMILES | COC(=O)C1=COC=C2C1=CC=C2C(=O)O | ||
| Standard InChIKey | KDGWNSIWYWYPGB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H8O5/c1-15-11(14)9-5-16-4-8-6(9)2-3-7(8)10(12)13/h2-5H,1H3,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
The herbs of Cerbera manghas L.
| Description | 1. Cerberic acids A possesses weak cytotoxic activity against HepG2, MCF-7, and HeLa cell lines with IC(50) values of 44.7, 52.3, 48.7 ug/ml, respectively. |

Cerberic acid Dilution Calculator

Cerberic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5413 mL | 22.7066 mL | 45.4133 mL | 90.8265 mL | 113.5332 mL |
| 5 mM | 0.9083 mL | 4.5413 mL | 9.0827 mL | 18.1653 mL | 22.7066 mL |
| 10 mM | 0.4541 mL | 2.2707 mL | 4.5413 mL | 9.0827 mL | 11.3533 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9083 mL | 1.8165 mL | 2.2707 mL |
| 100 mM | 0.0454 mL | 0.2271 mL | 0.4541 mL | 0.9083 mL | 1.1353 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Featured Products
- Cerbinal
Catalog No.:BCN4199
CAS No.:65597-42-4
- 4'-Demethylepipodophyllotoxin
Catalog No.:BCN5918
CAS No.:6559-91-7
- 1-Hydroxyacridone
Catalog No.:BCN7524
CAS No.:65582-54-9
- Europine N-oxide
Catalog No.:BCN1977
CAS No.:65582-53-8
- Cyclo(Pro-Ala)
Catalog No.:BCN2427
CAS No.:65556-33-4
- Esculentoside A
Catalog No.:BCN5010
CAS No.:65497-07-6
- Naftifine HCl
Catalog No.:BCC4806
CAS No.:65473-14-5
- (Z)-23-Coumaroylhederagenin
Catalog No.:BCN3748
CAS No.:654678-61-2
- Sitagliptin phosphate
Catalog No.:BCC9148
CAS No.:654671-78-0
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Fmoc-Asn(Trt)-ol
Catalog No.:BCC2585
CAS No.:654670-89-0
- Acantrifoic acid A
Catalog No.:BCN6488
CAS No.:654663-85-1
New Products
- Ophiopogonin D'
Catalog No.:BCN2645
CAS No.:65604-80-0
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- Fenretinide
Catalog No.:BCC1572
CAS No.:65646-68-6
- Esculentoside E
Catalog No.:BCN5014
CAS No.:65649-36-7
- Silybin B maltoside
Catalog No.:BCC8250
CAS No.:335299-49-5
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
- TC-C 14G
Catalog No.:BCC6144
CAS No.:656804-72-7
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- Metformin
Catalog No.:BCC9026
CAS No.:657-24-9
- H-Lys-OH.2HCl
Catalog No.:BCC2979
CAS No.:657-26-1
- H-Lys-OH.HCl
Catalog No.:BCC2978
CAS No.:657-27-2
Phenylpropionic acid derivates from the bark of Cerbera manghas.[Pubmed:20546844]
Fitoterapia. 2010 Oct;81(7):852-4.
Two new phenylpropionic acid derivates, Cerberic acids A (1) and B (2), were isolated from the bark of Cerbera manghas. Their structures were established on the basis of spectroscopic methods including IR, ESI-FT-ICR-MS, 1D and 2D NMR. Primary bioassays showed that compound 1 possessed weak cytotoxic activity against HepG2, MCF-7, and HeLa cell lines with IC(50) values of 44.7, 52.3, 48.7 mug/ml, respectively.
Cerberic acid,65597-44-6,Natural Products, buy Cerberic acid , Cerberic acid supplier , purchase Cerberic acid , Cerberic acid cost , Cerberic acid manufacturer , order Cerberic acid , high purity Cerberic acid


