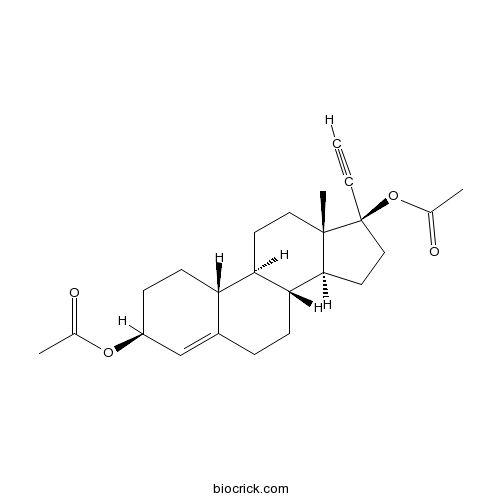
Ethynodiol diacetateCAS# 297-76-7 |
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Altrenogest
Catalog No.:BCC4479
CAS No.:850-52-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 297-76-7 | SDF | Download SDF |
| PubChem ID | 9270 | Appearance | Powder |
| Formula | C24H32O4 | M.Wt | 384.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ethynodiol acetate | ||
| Solubility | DMSO : ≥ 39 mg/mL (101.43 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [(3S,8R,9S,10R,13S,14S,17R)-17-acetyloxy-17-ethynyl-13-methyl-2,3,6,7,8,9,10,11,12,14,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate | ||
| SMILES | CC(=O)OC1CCC2C3CCC4(C(C3CCC2=C1)CCC4(C#C)OC(=O)C)C | ||
| Standard InChIKey | ONKUMRGIYFNPJW-KIEAKMPYSA-N | ||
| Standard InChI | InChI=1S/C24H32O4/c1-5-24(28-16(3)26)13-11-22-21-8-6-17-14-18(27-15(2)25)7-9-19(17)20(21)10-12-23(22,24)4/h1,14,18-22H,6-13H2,2-4H3/t18-,19-,20+,21+,22-,23-,24-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ethynodiol diacetate is a steroidal progestin which is used as a hormonal contraceptive, it has relatively little or no potency as an androgen,has significant estrogenic effects. |

Ethynodiol diacetate Dilution Calculator

Ethynodiol diacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6007 mL | 13.0036 mL | 26.0071 mL | 52.0143 mL | 65.0178 mL |
| 5 mM | 0.5201 mL | 2.6007 mL | 5.2014 mL | 10.4029 mL | 13.0036 mL |
| 10 mM | 0.2601 mL | 1.3004 mL | 2.6007 mL | 5.2014 mL | 6.5018 mL |
| 50 mM | 0.052 mL | 0.2601 mL | 0.5201 mL | 1.0403 mL | 1.3004 mL |
| 100 mM | 0.026 mL | 0.13 mL | 0.2601 mL | 0.5201 mL | 0.6502 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ethynodiol diacetate is one of the first synthetic progestogens used in contraceptive pills.
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- (E)-N-Caffeoylputrescine
Catalog No.:BCC8391
CAS No.:29554-26-5
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- Olivil
Catalog No.:BCN5198
CAS No.:2955-23-9
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
- MNI-caged-L-glutamate
Catalog No.:BCC7086
CAS No.:295325-62-1
- Valerosidate
Catalog No.:BCN6750
CAS No.:29505-31-5
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
Biotransformation of oral contraceptive ethynodiol diacetate with microbial and plant cell cultures.[Pubmed:23021311]
Chem Cent J. 2012 Sep 29;6(1):109.
UNLABELLED: BACKGROUND: Biotransformation by using microbial and plant cell cultures has been applied effectively for the production of fine chemicals on large scale. Inspired by the wealth of literature available on the biotransformation of steroids, we decided to investigate the biotransformation of Ethynodiol diacetate (1) by using plant and microbial cultures. RESULTS: The biotransformation of Ethynodiol diacetate (1) with Cunninghamella elegans and plant cell suspension cultures of Ocimum basilicum and Azadirachta indica is being reported here for the first time. Biotransformation of 1 with Cunninghamella elegans yielded three new hydroxylated compounds, characterized as 17alpha-ethynylestr-4-en-3beta,17beta-diacetoxy-6alpha-ol (2), 17alpha-ethynylestr-4-en-3beta,17beta-diacetoxy-6beta-ol (3), and 17alpha-ethynylestr-4-en-3beta,17beta-diacetoxy-10beta-ol (4) and a known metabolite, 17alpha-ethynyl-17beta-acetoxyestr-4-en-3-one (5). The biotransformation of 1 with Ocimum basilicum included hydrolysis of the ester group, oxidation of alcohol into ketone, and rearrangement of the hydroxyl group. Thus four major known metabolites were characterized as 17alpha-ethynyl-17beta-acetoxyestr-4-en-3-one (5), 17alpha-ethynyl-17beta-hydroxyestr-4-en-3-one (6), 17alpha-ethynyl-3 beta-hydroxy-17beta-acetoxyestr-4-ene (7) and 17alpha-ethynyl-5alpha,17beta-dihydroxyestr-3-ene (8). Biotransformation of 1 with Azadirachta indica culture yielded compounds 5 and 6. Spectroscopic data of compound 8 is being reported for the first time. Structure of compound 6 was unambiguously deduced through single-crystal x-ray diffraction studies. CONCLUSION: Biotransformation of an oral contraceptive, Ethynodiol diacetate (1), by using microbial and plant cell cultures provides an efficient route to the synthesis of a library of new steroids with potential contraceptive properties. These methods can be employed in the production of such compounds with high stereoselectivity.
Estimation of impurity profiles of drugs and related materials: part XXI. HPLC/UV/MS study of the impurity profile of ethynodiol diacetate.[Pubmed:12110402]
J Pharm Biomed Anal. 2002 Aug 1;29(6):1153-7.
The impurity profile of Ethynodiol diacetate was investigated using the HPLC/UV/MS method. Using the slightly modified HPLC method of USP 24 two impurities, earlier isolated by preparative HPLC and investigated by NMR spectroscopy were separated and characterised. The mass spectra amended by the diode-array UV spectra supported the earlier found structures (E and Z isomers of 17alpha-ethinyl-estr-4-ene-3beta,17-diol-3-acetate-17-(3'-acetoxy-2'-butenoate). Another, hitherto not described impurity, 17alpha-ethinyl-estr-4-ene-3beta,17-diol-3-acetate-17-(3-oxo-butanoate) has also been separated and characterised by means of its mass spectrum, NMR and UV spectra.
Protective role of nordihydroguaiaretic acid (NDGA) against the genotoxic damage induced by ethynodiol diacetate in human lymphocytes in vitro.[Pubmed:17915765]
J Environ Biol. 2007 Apr;28(2):279-82.
Antioxidants and plant products are reported to reduce the genotoxic damage of steroids. In our present study we have tested different dosages of nordihydroguaiaretic acid (NDGA) against the genotoxic damage induced by Ethynodiol diacetate in the presence of S9 mix. Treatments with nordihydroguaiaretic acid (NDGA) results in the reduction of the genotoxic damage. A significant decrease was observed at all the tested doses of NDGA in sister chromatic exchanges of number of abnormal cells. The results suggest a protective role of NDGA against the genotoxic damage.
Influence of Ethynodiol Diacetate on the Formation of A-Homo-3Oxa-5?-Pregnane-4,20-Dione in Female Rats.[Pubmed:10196509]
Endocr Regul. 1998 Sep;32(3):125-131.
OBJECTIVE: To give more insight in the progesterone metabolism in rat after the treatment with the progestin Ethynodiol diacetate. METHODS: Urinary excretion of the metabolites of subcutaneously administred (4-14C)-progesterone was studied in female rats. After an acid hydrolysis and extraction of urine the metabolites were analysed by thin layer chromatography and by gas chromatography-mass spectrometry. RESULTS: The most of radioactivity was excreted during the first 24 h, and total of 8.36 % has been recovered within four days. The excreted metabolites in urine were found as glucuronides and free steroids (80.72 %), and 19.28 % were determined as sulphates. Among detected metabolites, 5alpha-pregnane-3,20-dione, 3alpha-hydroxy-5alpha-pregnan-20-one and A-homo-3-oxa-5alpha-pregnane-4,20-dione were determined in the urinary extracts. The last one has not yet been identified before in rat urine. CONCLUSIONS: Consecutive injections of progestin Ethynodiol diacetate (6 mg/kg b.w. daily) to adult female rats during 10 days (short-term treatment), or during 70 days (long-term treatment), starting on the 21st day of life, caused significant differences in the amounts of excreted 3alpha-hydroxy-5alpha-pregnan-20-one and A-homo-3-oxa-5alpha-pregnane-4,20-dione. Significant increase in the weights of pituitary, liver and kidneys were noted in rats treated with Ethynodiol diacetate. The short-term treatment caused an increase, while after the long-term treatment a decrease of the ovarian weight was observed.


