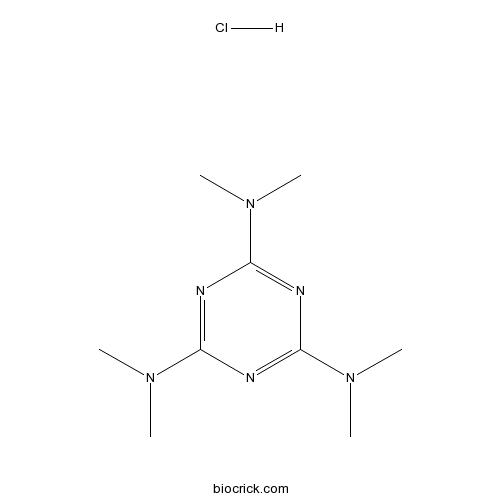
Altretamine hydrochlorideCAS# 2975-00-0 |
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Misoprostol
Catalog No.:BCC5240
CAS No.:59122-46-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2975-00-0 | SDF | Download SDF |
| PubChem ID | 165057 | Appearance | Powder |
| Formula | C9H19ClN6 | M.Wt | 246.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-N,2-N,4-N,4-N,6-N,6-N-hexamethyl-1,3,5-triazine-2,4,6-triamine;hydrochloride | ||
| SMILES | CN(C)C1=NC(=NC(=N1)N(C)C)N(C)C.Cl | ||
| Standard InChIKey | AKAQBNIEQUSIJL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H18N6.ClH/c1-13(2)7-10-8(14(3)4)12-9(11-7)15(5)6;/h1-6H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Altretamine hydrochloride Dilution Calculator

Altretamine hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0528 mL | 20.2642 mL | 40.5285 mL | 81.057 mL | 101.3212 mL |
| 5 mM | 0.8106 mL | 4.0528 mL | 8.1057 mL | 16.2114 mL | 20.2642 mL |
| 10 mM | 0.4053 mL | 2.0264 mL | 4.0528 mL | 8.1057 mL | 10.1321 mL |
| 50 mM | 0.0811 mL | 0.4053 mL | 0.8106 mL | 1.6211 mL | 2.0264 mL |
| 100 mM | 0.0405 mL | 0.2026 mL | 0.4053 mL | 0.8106 mL | 1.0132 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Altretamine is an alkylating agent proposed as an antineoplastic.
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Threo-methylphenidate hydrochloride
Catalog No.:BCC5818
CAS No.:298-59-9
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
- Nitrotetrazolium Blue chloride
Catalog No.:BCC6465
CAS No.:298-83-9
- MTT
Catalog No.:BCC8031
CAS No.:298-93-1
- Desmethylbellidifolin
Catalog No.:BCN3868
CAS No.:2980-32-7
- H-Pro-NMe2
Catalog No.:BCC3019
CAS No.:29802-22-0
Doxorubicin-hexamethylmelamine therapy of small cell carcinoma of the lung.[Pubmed:6271385]
Cancer. 1981 Nov 1;48(9):1944-6.
Eighteen patients with small cell carcinoma of the lung treated with doxorubicin hydrochloride and hexamethylmelamine are presented. Fifteen of these patients had extensive disease at presentation. Four patients in this group died after one or fewer courses of chemotherapy. The median duration of survival for the entire group of patients is 15 months. Six patients are alive from 18 to 56 months without evidence of disease. Drug toxicity was minimal and well tolerated, which permitted this regimen to be given in an outpatient setting.
Ovarian cancer. Effective treatment after alkylating-agent failure.[Pubmed:107337]
JAMA. 1979 May 4;241(18):1908-11.
Combination chemotherapy consisting of hexamethylmelamine and cisplatin, alone or with doxorubicin hydrochloride, was given to 27 patients with advanced ovarian cancer who had disease progression with therapy including alkylating agents. Eighteen (67%) had greater than 50% regression of measurable disease or disease that could be evaluated but not measured, for a projected median duration of seven months. The projected median survival for all patients is nine months from the time of entry into the study and 33 months from the time of diagnosis of ovarian cancer. The treatment could be readily administered on an outpatient basis with a regimen of hydration and diuresis that nearly completely prevented platinum-induced renal tubular damage. Myelosuppression was severe in 11 patients (40%), but there was no treatment-related deaths. Agents of such high activity should be considered as components of initial therapy for stage III and IV cancers.
Hexamethylmelamine and hexamethylmelamine hydrochloride.[Pubmed:6808457]
Pharm Weekbl Sci. 1982 Apr 23;4(2):25-31.
Methods of analysis of the anticancer drug hexamethylmelamine, its physicochemical properties, its therapeutic uses and its fate in vivo upon oral administration, are described. The data are taken in part from the literature, in part from our own studies. The low solubility of hexamethylmelamine in water has prevented the parenteral administration of the drug to humans. The preparation of the water-soluble monohydrochloride of hexamethylmelamine, which may be suitable for this purpose, is reported, as well as its physicochemical characteristics and methods of analysis.
Comparison of DNA damage by methylmelamines and formaldehyde.[Pubmed:6788992]
J Natl Cancer Inst. 1981 Jul;67(1):217-21.
The cytoxicity and DNA damaging activity of S9-activated hexamethylmelamine (HMM) and pentamethylmelamine (PMM) were compared with suspected active metabolites in mouse leukemia L1210 cells. The presence of semicarbazide hydrochloride did not alter the cytotoxicity of S9-activated HMM and PMM or that of their hydroxylated analogs monomethylolpentamethylmelamine (MPM) and trimethyloltrimethylmelamine (TTM), which have been suggested as active metabolites. Following treatment of L1210 cells with high concentrations of activated HMM and PMM, there were no DNA single-strand breaks or interstrand cross-links observed by DNA alkaline elution and only a low frequency of DNA-protein cross-links. Formaldehyde (FA) at nonlethal concentrations caused far greater DNA-protein cross-linking. In contrast, the polyfunctional TTM produced both DNA-protein cross-linking and DNA interstrand cross-linking. The cytotoxicities of HMM and PMM were found unlikely to be related to extracellular or intracellular release of FA.


