AltenueneCAS# 29752-43-0 |
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
Quality Control & MSDS
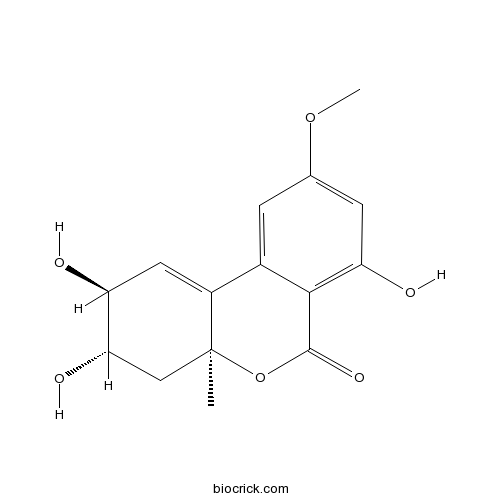
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29752-43-0 | SDF | Download SDF |
| PubChem ID | 34687 | Appearance | Powder |
| Formula | C15H16O6 | M.Wt | 292.28 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S,4aS)-2,3,7-trihydroxy-9-methoxy-4a-methyl-3,4-dihydro-2H-benzo[c]chromen-6-one | ||
| SMILES | CC12CC(C(C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O | ||
| Standard InChIKey | MMHTXEATDNFMMY-WBIUFABUSA-N | ||
| Standard InChI | InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Altenuene is a mycotoxin, it frequently occurs in food and feed items infested by fungi of the genus Alternaria. 2. Altenuene exhibits cytotoxic activity against lung cancer cell line A549, breast cancer cell line MDA-MB-231 and pancreatic cancer cell line PANC-1. 3. Altenuene demonstrates moderate activity against Staphylococcus aureus. |
| Targets | Antifection |

Altenuene Dilution Calculator

Altenuene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4214 mL | 17.1069 mL | 34.2138 mL | 68.4275 mL | 85.5344 mL |
| 5 mM | 0.6843 mL | 3.4214 mL | 6.8428 mL | 13.6855 mL | 17.1069 mL |
| 10 mM | 0.3421 mL | 1.7107 mL | 3.4214 mL | 6.8428 mL | 8.5534 mL |
| 50 mM | 0.0684 mL | 0.3421 mL | 0.6843 mL | 1.3686 mL | 1.7107 mL |
| 100 mM | 0.0342 mL | 0.1711 mL | 0.3421 mL | 0.6843 mL | 0.8553 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Threo-methylphenidate hydrochloride
Catalog No.:BCC5818
CAS No.:298-59-9
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
- Nitrotetrazolium Blue chloride
Catalog No.:BCC6465
CAS No.:298-83-9
- MTT
Catalog No.:BCC8031
CAS No.:298-93-1
- Desmethylbellidifolin
Catalog No.:BCN3868
CAS No.:2980-32-7
- H-Pro-NMe2
Catalog No.:BCC3019
CAS No.:29802-22-0
- Fatostatin A
Catalog No.:BCC6184
CAS No.:298197-04-3
Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp.[Pubmed:22061662]
Fitoterapia. 2012 Jan;83(1):209-14.
Two new polyketides, 7-hydroxy-3, 5-dimethyl-isochromen-1-one (1) and 6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione (2), along with eleven known compounds, 5'-methoxy-6-methyl-biphenyl-3,4,3'-triol (3), 7-hydroxy-3-(2-hydroxy-propyl)-5-methyl-isochromen-1-one (4), rubralactone (5), isoAltenuene (6), Altenuene (7), dihydroAltenuenes A (8), altenusin (9), alterlactone (10), 6-O-methylnorlichexanthone (11), norlichexanthone (12), and griseoxanthone C (13) were isolated from the culture of the endolichenic fungus Ulocladium sp. Compound 2 was obtained as a racemate with an unprecedented chemical skeleton. The NMR data assignments for 3 and 4 were achieved for the first time. Compounds 1-13 were screened for their antimicrobial and radical scavenging activities. Compound 1 showed some antifungal activity against Candida albicans SC 5314 with IC(50) of 97.93 +/- 1.12 muM. Compounds 11-13 showed strong activity against Bacillus subtilis with IC(50) in the range of 1-5 muM. Compound 12 significantly inhibited the growth of methicillin-resistant Staphylococcus aureus with IC(50) of 20.95 +/- 1.56 muM. Compounds 9 and 10 showed strong radical scavenging activity in comparison with vitamin C. The plausible biosynthetic pathways for compounds 1, 2, and 4-8 were discussed.
Alternaria toxins in South African sunflower seeds: cooperative study.[Pubmed:28755328]
Mycotoxin Res. 2017 Nov;33(4):309-321.
Sunflower seed samples (N = 80) from different sunflower cultivars originating from different localities in South Africa were analyzed for 15 toxins produced by fungi of the genus Alternaria by means of a simple one-step extraction dilute-and-shoot HPLC-MS/MS approach. References for valine-tenuazonic acid (Val-TeA), altenusin (ALTS), and altenuisol (ALTSOH) were isolated from fungal culture extracts and spectroscopically characterized. Additionally, valine-tenuazonic acid was tested regarding its cytotoxicity in comparison with tenuazonic acid (TeA) and showed less activity on HT-29 cells. Furthermore, alternariol monomethyl ether-3-O-ss-D-glucoside (AME-3G) was produced by fermentation of alternariol monomethyl ether (AME) with the fungus Rhizopus oryzae. The seed samples were analyzed both with and without hulls. The method covers the AAL toxins TA1 and TA2, Altenuene (ALT) and iso-Altenuene (iso-ALT), altenuisol, altenusin, altertoxin I (ATX-I) and altertoxin II (ATX-II), alternariol (AOH) and alternariol monomethyl ether, alternariol monomethyl ether-3-O-ss-D-glucoside, tenuazonic acid, allo-tenuazonic acid (allo-TeA) and valine-tenuazonic acid, and tentoxin (TEN). More than 80% of the samples were positive for one or more analytes above the respective limit of detection (0.2-23 mug/kg). Alternariol, its monomethyl ether, tentoxin, tenuazonic acid, altenuisol, and valine-tenuazonic acid were found in quantifiable amounts. The highest prevalences were found for tentoxin (73% positive, mean content 13.2 mug/kg, maximum level 130 +/- 0.9 mug/kg) followed by tenuazonic acid (51% positive, mean content 630 mug/kg, maximum level 6300 +/- 560 mug/kg). The obtained data were further analyzed statistically to identify quantitative or qualitative relationships between the levels of Alternaria toxin in the samples.
[A new sesquiterpenoid from fungus Colletotrichum sp. and its cytotoxicity].[Pubmed:23984524]
Yao Xue Xue Bao. 2013 Jun;48(6):891-5.
A novel sesquiterpenoid (1) and three known compounds identified as isoAltenuene (2), Altenuene (3), and alternariol 4, 10-O-dimethyl ether (4), were isolated in our investigation of the cytotoxic constituents from solid cultures of the endophytic fungus Colletotrichum sp. The structures of these compounds were elucidated through spectroscopic data analysis. All compounds exhibited cytotoxic activity against lung cancer cell line A549, breast cancer cell line MDA-MB-231 and pancreatic cancer cell line PANC-1. Compound 4 could induce the PANC-1 cells inflation or death, but couldn't induce apoptosis at the IC50 of 60.2 microg x mL(-1).
A new alternariol glucoside from fungus Alternaria alternate cib-137.[Pubmed:25520187]
Nat Prod Res. 2015;29(9):848-52.
A new secondary metabolite, 2-O-methylalternariol 4-O-beta-[4-methoxyl-glucopyranoside] (1), together with four known compounds alternariol methyl ether (2), Altenuene (3), isoAltenuene (4) and 2-(2'S-hydroxypropyl)-5-methyl-7-hydroxychromone (5), was isolated from the fungus Alternaria alternate cib-137. Its structure was elucidated on the basis of spectroscopic data. Compounds 3 and 4 demonstrated moderate activity against Staphylococcus aureus.


