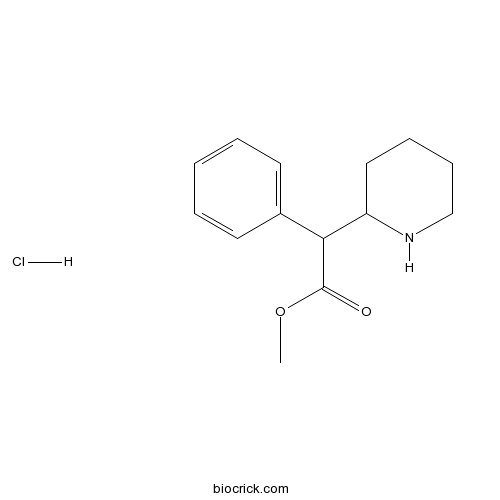
Threo-methylphenidate hydrochlorideInhibitor of dopamine and noradrenalin transporters. Psychomotor stimulant CAS# 298-59-9 |
- Lomeguatrib
Catalog No.:BCC1133
CAS No.:192441-08-0
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 298-59-9 | SDF | Download SDF |
| PubChem ID | 9280 | Appearance | Powder |
| Formula | C14H20ClNO2 | M.Wt | 269.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ritalin | ||
| Solubility | Soluble to 50 mM in water and to 100 mM in ethanol | ||
| Chemical Name | Threo-methyl α-Phenyl-2-piperidineacetate hydrochloride | ||
| SMILES | [H+].[Cl-].COC(=O)C(C1CCCCN1)c2ccccc2 | ||
| Standard InChIKey | JUMYIBMBTDDLNG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H19NO2.ClH/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12;/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Psychomotor stimulant. Inhibitor of dopamine and noradrenalin transporters that increases the extracellular concentration of dopamine and noradrenalin. Increases locomotor activity in vivo. |

Threo-methylphenidate hydrochloride Dilution Calculator

Threo-methylphenidate hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7069 mL | 18.5343 mL | 37.0686 mL | 74.1372 mL | 92.6715 mL |
| 5 mM | 0.7414 mL | 3.7069 mL | 7.4137 mL | 14.8274 mL | 18.5343 mL |
| 10 mM | 0.3707 mL | 1.8534 mL | 3.7069 mL | 7.4137 mL | 9.2672 mL |
| 50 mM | 0.0741 mL | 0.3707 mL | 0.7414 mL | 1.4827 mL | 1.8534 mL |
| 100 mM | 0.0371 mL | 0.1853 mL | 0.3707 mL | 0.7414 mL | 0.9267 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
- Nitrotetrazolium Blue chloride
Catalog No.:BCC6465
CAS No.:298-83-9
- MTT
Catalog No.:BCC8031
CAS No.:298-93-1
- Desmethylbellidifolin
Catalog No.:BCN3868
CAS No.:2980-32-7
- H-Pro-NMe2
Catalog No.:BCC3019
CAS No.:29802-22-0
- Fatostatin A
Catalog No.:BCC6184
CAS No.:298197-04-3
- Shanzhiside
Catalog No.:BCN5203
CAS No.:29836-27-9
- Astilbin
Catalog No.:BCN5204
CAS No.:29838-67-3
- Pirenzepine dihydrochloride
Catalog No.:BCC6923
CAS No.:29868-97-1
- Ro 67-7476
Catalog No.:BCC6145
CAS No.:298690-60-5
- Amygdalin
Catalog No.:BCN5206
CAS No.:29883-15-6
- Astringin
Catalog No.:BCN3412
CAS No.:29884-49-9
A post hoc analysis of d-threo-methylphenidate hydrochloride (focalin) versus d,l-threo-methylphenidate hydrochloride (ritalin).[Pubmed:15502601]
J Am Acad Child Adolesc Psychiatry. 2004 Nov;43(11):1415-21.
OBJECTIVE: To evaluate clinical measures of the benefit/risk ratio in a post hoc analysis of a clinical trial of d-Threo-methylphenidate hydrochloride (d-MPH) and d,l-Threo-methylphenidate hydrochloride (d,l-MPH). METHOD: Data from a phase III clinical trial was used to compare equimolar doses of d-MPH and d,l-MPH treatment for attention-deficit/hyperactivity disorder (ADHD) on clinician ratings of improvement/deterioration, teacher ratings of remission, and duration of action. RESULTS: d-MPH was clinically and statistically significantly superior to d,l-MPH on clinician's dimensional ratings of global improvement, teacher ratings of remission of ADHD symptoms and parent 6:00 p.m. ADHD symptom ratings. No treatment differences were observed for symptom ratings at 3:00 p.m. and for 6:00 p.m. math test performance. CONCLUSION: The results suggest that the two drugs may have distinct profiles on the measures analyzed. Because d-MPH may have be more than twice the potency of d,l-MPH, the differences reported here are only applicable to the doses of the study drugs involved in the clinical trial. The results are limited by the exploratory nature of our analysis, small samples, and multiple analyses. The findings are suggestive that further study is warranted to look at these differences prospectively but cannot be used to draw clinical conclusions at this time.
Comparative pharmacodynamics and plasma concentrations of d-threo-methylphenidate hydrochloride after single doses of d-threo-methylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in a double-blind, placebo-controlled, crossover laboratory school study in children with attention-deficit/hyperactivity disorder.[Pubmed:15502602]
J Am Acad Child Adolesc Psychiatry. 2004 Nov;43(11):1422-9.
OBJECTIVE: Methylphenidate has four optical isomers due to two asymmetries (erythro-threo and dextro-levo). The initial commercial formulation eliminated the erythro isomer, but the dextro-levo asymmetry was racemic, with equal amounts of d and l-threo isomers (d,l-MPH). Previous work has suggested that the d-threo isomer methylphenidate (d-MPH) rather than the l-threo isomer (l-MPH) is responsible for the clinical effects in children with attention-deficit/hyperactivity disorder (ADHD). This study compared the efficacy of acute equimolar doses of d-MPH and dl-MPH in reducing ADHD symptoms over an 8-hour period in a laboratory school setting and investigated the relationship of efficacy to plasma levels of MPH. METHOD: Thirty-two children with ADHD enrolled in this double-blind, placebo-controlled, crossover study, and 31 completed the study. On seven separate occasions separated by at least 6 days, the children received a single morning dose of d-MPH (2.5, 5, or 10 mg), d,l-MPH (5, 10, or 20 mg), or placebo and then were observed in a laboratory classroom setting for 8 hours. At specified intervals, blinded observers rated behavior, and the children performed a computerized math test. The plasma levels of MPH were related to the response to study medication. The safety profiles of the two formulations were compared. RESULTS: For both formulations, the responses to both MPH preparations were dose related, the plasma concentrations of l-MPH were negligible and of d-MPH were indistinguishable, and clinical efficacy was highly correlated with plasma concentrations of d-MPH. The efficacy of the d-isomer was equivalent to the racemic preparation in reducing ADHD symptoms and increasing academic productivity. CONCLUSIONS: The efficacy of MPH resides in the d-isomer. The elimination of the l-isomer does not diminish the efficacy of an acute dose of methylphenidate.
A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder.[Pubmed:15502600]
J Am Acad Child Adolesc Psychiatry. 2004 Nov;43(11):1406-14.
OBJECTIVE: To evaluate the efficacy and safety of dexmethylphenidate hydrochloride (d-MPH, Focalin) for the treatment of attention-deficit/hyperactivity disorder (ADHD) and to test an a priori hypothesis that d-MPH would have a longer duration of action than d,l-threo-methylphenidate (d,l-MPH). METHOD: This was a randomized, double-blind study conducted at 12 U.S. centers. One hundred thirty-two subjects received d-MPH (n=44), d,l-MPH (n=46), or placebo (n=42) twice daily for 4 weeks, with titration of the dose based on weekly clinic visits. The primary efficacy variable was change from baseline to last study visit on teacher-completed Swanson, Nolan, and Pelham Rating Scale (Teacher SNAP). Secondary efficacy measures included the change on parent-completed SNAP (Parent SNAP), Clinical Global Impressions Scale-Improvement (CGI-I) score, and Math Test performance. Assessments at home in late afternoon were included to test the hypothesis that d-MPH would have a longer duration of efficacy than d,l-MPH. Safety was assessed through monitoring occurrence and severity of adverse events and discontinuations related to them. RESULTS: Treatment with either d-MPH (p=.0004) or d,l-MPH (p=.0042) significantly improved Teacher SNAP ratings compared with placebo. The d-MPH group showed significant improvements compared with placebo on the afternoon Parent SNAP ratings (p=.0003) and scores on the Math Test (p=.0236) obtained late in the afternoon at 6:00 p.m. Sixty-seven percent of patients showed improvement on d-MPH and 49% on d,l-MPH based on CGI-I scores. Both d-MPH and d,l-MPH were well tolerated, no patient in the d-MPH group and only two patients each in the d,l-MPH and placebo groups discontinued the study. CONCLUSIONS: For the treatment of ADHD, an average titrated dose of 18.25 mg/day of d-MPH is as efficacious and safe as an average titrated dose of 32.14 mg/day of d,l-MPH. Both active treatments have large effect sizes. Thus, d-MPH and d,l-MPH appear to provide similar efficacy, and d-MPH may have longer duration of action after twice-daily dosing, but additional studies are needed to determine the statistical and clinical significance of this possibility.
Methylphenidate (Ritalin): behavioral studies in the rat.[Pubmed:17454243]
Int J Neurosci. 2007 Jun;117(6):757-94.
Attention Deficit Hyperactivity Disorder (ADHD) is a neuropsychiatric syndrome with an onset in childhood characterized by an inability to remain focused or to concentrate for prolonged periods of time. Children suffering from this disease are many times described as either inattentive or as hyperactive-impulsive depending on what form of the disease they manifest. Methylphenidate is the preferred treatment for this behavioral disorder and is used for long term disease management. Much still remains unknown concerning this stimulant and its effects on behavior and future abuse potential are pertinent questions. Since animal models are used to study the mechanism of drug action and rats are used often in drug studies, the objective of this review is to summarize the research reports that mainly have used rats as the model to investigate the action of methylphenidate. Topics discussed in this review include: (1) What effect does a single dose of methylphenidate have on locomotion activity; (2) Does repeated administration of methylphenidate result in tolerance or sensitization; and (3) Does methylphenidate have rewarding properties as measured by the self-administration and condition placed preference paradigms.


