Nitrotetrazolium Blue chlorideCAS# 298-83-9 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 298-83-9 | SDF | Download SDF |
| PubChem ID | 9281 | Appearance | Powder |
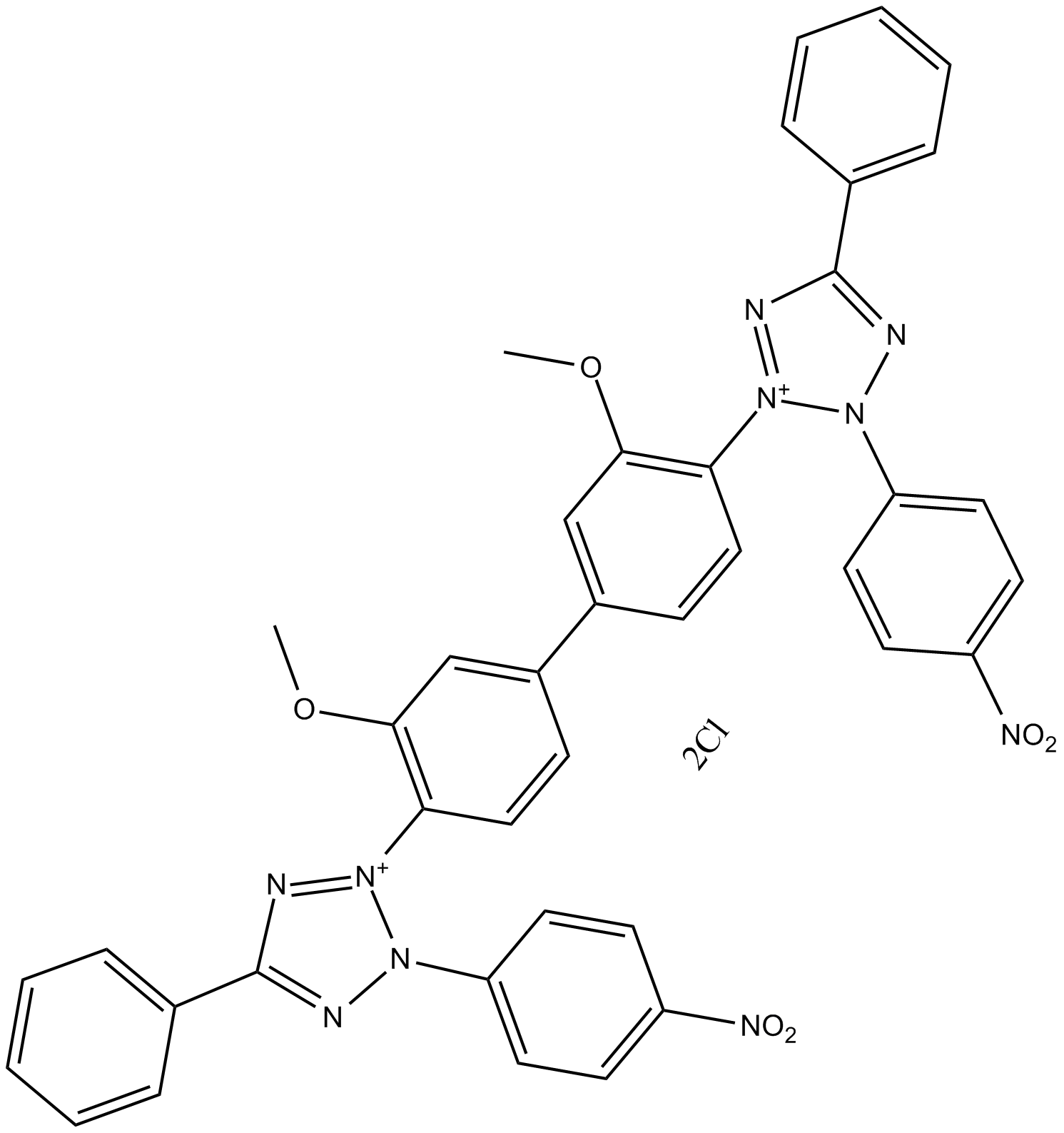
| Formula | C40H30Cl2N10O6 | M.Wt | 817.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Nitro BT; p-Nitrotetrazolium blue; Nitrotetrazolium Blue chloride | ||
| Solubility | >40.9mg/ml in DMSO | ||
| Chemical Name | 2-[2-methoxy-4-[3-methoxy-4-[3-(4-nitrophenyl)-5-phenyltetrazol-2-ium-2-yl]phenyl]phenyl]-3-(4-nitrophenyl)-5-phenyltetrazol-2-ium;dichloride | ||
| SMILES | COC1=C(C=CC(=C1)C2=CC(=C(C=C2)[N+]3=NC(=NN3C4=CC=C(C=C4)[N+](=O)[O-])C5=CC=CC=C5)OC)[N+]6=NC(=NN6C7=CC=C(C=C7)[N+](=O)[O-])C8=CC=CC=C8.[Cl-].[Cl-] | ||
| Standard InChIKey | FSVCQIDHPKZJSO-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C40H30N10O6.2ClH/c1-55-37-25-29(13-23-35(37)47-43-39(27-9-5-3-6-10-27)41-45(47)31-15-19-33(20-16-31)49(51)52)30-14-24-36(38(26-30)56-2)48-44-40(28-11-7-4-8-12-28)42-46(48)32-17-21-34(22-18-32)50(53)54;;/h3-26H,1-2H3;2*1H/q+2;;/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NBT(Nitro BT;p-Nitrotetrazolium blue) is a substrate for dehydrogenases; is used with the alkaline phosphatase substrate 5-Bromo- 4-Chloro-3-Indolyl Phosphate (BCIP) in western blotting and immunohistological staining procedures. |

Nitrotetrazolium Blue chloride Dilution Calculator

Nitrotetrazolium Blue chloride Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nitrotetrazolium Blue chloride
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
- Threo-methylphenidate hydrochloride
Catalog No.:BCC5818
CAS No.:298-59-9
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- MTT
Catalog No.:BCC8031
CAS No.:298-93-1
- Desmethylbellidifolin
Catalog No.:BCN3868
CAS No.:2980-32-7
- H-Pro-NMe2
Catalog No.:BCC3019
CAS No.:29802-22-0
- Fatostatin A
Catalog No.:BCC6184
CAS No.:298197-04-3
- Shanzhiside
Catalog No.:BCN5203
CAS No.:29836-27-9
- Astilbin
Catalog No.:BCN5204
CAS No.:29838-67-3
- Pirenzepine dihydrochloride
Catalog No.:BCC6923
CAS No.:29868-97-1
- Ro 67-7476
Catalog No.:BCC6145
CAS No.:298690-60-5
- Amygdalin
Catalog No.:BCN5206
CAS No.:29883-15-6
- Astringin
Catalog No.:BCN3412
CAS No.:29884-49-9
- Sparteine sulfate pentahydrate
Catalog No.:BCN1267
CAS No.:299-39-8
- Eledoisin-Related Peptide
Catalog No.:BCC5849
CAS No.:2990-43-4
Astragaloside IV Protects Rat Cardiomyocytes from Hypoxia-Induced Injury by Down-Regulation of miR-23a and miR-92a.[Pubmed:30257251]
Cell Physiol Biochem. 2018;49(6):2240-2253.
BACKGROUND/AIMS: Astragaloside IV (AS-IV), a traditional Chinese medicine isolated from Astragalus membranaceus, has been shown to exert cardioprotective effect previously. This study aimed to reveal the effects of AS-IV on hypoxia-injured cardiomyocyte. METHODS: H9c2 cells were treated with various doses of AS-IV for 24 h upon hypoxia. CCK-8 assay, flow cytometry/Western blot, and qRT-PCR were respectively conducted to measure the changes in cell viability, apoptosis, and the expression of miR-23a and miR-92a. Sprague-Dawley rats were received coronary ligation, and were administrated by various doses of AS-IV for 14 days. The infarct volume and outcome of rats followed by ligation were tested by ultrasound, arteriopuncture and Nitrotetrazolium Blue chloride (NBT) staining. RESULTS: We found that 10 mug/ml of AS-IV exerted myocardioprotective effects against hypoxia-induced cell damage, as AS-IV significantly increased H9c2 cells viability and decreased apoptosis. Interestingly, the myocardioprotective effects of AS-IV were alleviated by miR-23a and/or miR-92a overexpression. Knockdown of miR-23a and miR-92a activated PI3K/AKT and MAPK/ ERK signaling pathways. Bcl-2 was a target gene for miR-23a, and BCL2L2 was a target gene for miR-92a. In the animal model of myocardial infarction (MI), AS-IV significantly reduced the infarct volume, ejection fraction (EF), shortening fraction (FS) and LV systolic pressure (LVSP), and significantly increased left ventricular end-diastolic internal diameter (LVEDd). And also, the elevated expression of miR-23a and miR-92a in MI rat was reduced by AS-IV. CONCLUSION: AS-IV protected cardiomyocytes against hypoxia-induced injury possibly via down-regulation of miR-23a and miR-92a, and via activation of PI3K/AKT and MAPK/ERK signaling pathways.
Copper(II) complexes with Fusobacterium nucleatum adhesin FadA: Coordination pattern, physicochemical properties and reactivity.[Pubmed:30243120]
J Inorg Biochem. 2018 Dec;189:69-80.
Fusobacterium nucleatum is an anaerobic, Gram-negative bacteria linked to colon cancer. It is interesting to determine how metal ions interact with bacterial adhesin proteins. To this end, the coordination of ATDAAS-NH2 and MKKFL-NH2 fragments of Fusobacterium adhesin A (FadA) to copper(II) ions was studied by potentiometry, spectroscopic techniques (UV-Vis, CD, EPR and NMR) and the density functional theory (DFT) methods. At pH6.8 (colon physiological pH), the metal ion in the first peptide (ATDAAS-NH2) is coordinated by one oxygen and three nitrogen donors while in the second one (MKKFL-NH2) - by sulfur and three nitrogen atoms. Both complexes form two five- and one six-membered stable chelate rings. Moreover, reactivity studies confirmed the production of reactive oxygen species such as hydroxyl radical, superoxide anion radical and singlet oxygen. Generation of reactive oxygen species (ROS) was observed during gel electrophoresis and spectroscopic assays with reporting molecules like NDMA (N,N-dimethyl-p-nitrosoaniline) and NBT (Nitrotetrazolium Blue chloride). All reactions were conducted in the presence of hydrogen peroxide as endogenous oxidant.
Synthesis, Structure, DNA Interaction, and SOD Activity of Three Nickel(II) Complexes Containing L-Phenylalanine Schiff Base and 1,10-Phenanthroline.[Pubmed:30073020]
Bioinorg Chem Appl. 2018 Jul 5;2018:8478152.
Three hexacoordinated octahedral nickel(II) complexes, [Ni(sal-L-phe)(phen)(CH3OH)].CH3OH (1), [Ni(naph-L-phe)(phen)(CH3OH)] (2), and [Ni(o-van-L-phe)(phen)(CH3OH)].5CH3OH (3) (sal-L-phe = a Schiff base derived from salicylaldehyde and L-phenylalanine, naph-L-phe = a Schiff base derived from 2-hydroxy-1-naphthaldehyde and L-phenylalanine, o-van-L-phe = a Schiff base derived from o-vanillin and L-phenylalanine, and phen = 1,10-phenanthroline), have been synthesized and characterized by elemental analysis, IR spectra, and single-crystal X-ray diffraction. The interactions of these complexes with CT-DNA were studied by UV-Vis absorption spectroscopy, fluorescence spectroscopy, circular dichroism spectroscopy, and viscosity measurements. The binding constant (Kb) values of 1.82 x 10(4) M(-1) for 1, 1.96 x 10(4) M(-1) for 2, and 2.02 x 10(4) M(-1) for 3 suggest that each of these complexes could bind with DNA in a moderate intercalative mode. Complex 3 exhibited a stronger interaction with CT-DNA than complexes 1 and 2. In addition, the superoxide scavenging activity of these complexes was investigated by the Nitrotetrazolium Blue chloride (NBT) light reduction method, and the results showed that they exhibited a significant superoxide scavenging activity with the IC50 values of 4.4 x 10(-5) M for complex 1, 5.6 x 10(-5) M for complex 2, and 3.1 x 10(-5) M for complex 3, respectively.
Pollutant-induced cell death and reactive oxygen species accumulation in the aerial roots of Chinese banyan (Ficus microcarpa).[Pubmed:27805029]
Sci Rep. 2016 Nov 2;6:36276.
Industrial pollutants induce the production of toxic reactive oxygen species (ROS) such as O2(.-), H2O2, and (.)OH in plants, but they have not been well quantified or localized in tissues and cells. This study evaluated the pollutant- (HSO3(-), NH4NO3, Al(3+), Zn(2+), and Fe(2+)) induced toxic effects of ROS on the aerial roots of Chinese banyan (Ficus microcarpa). Root cell viability was greatly reduced by treatment with 20 mM NaHSO3, 20 mM NH4NO3, 0.2 mM AlCl3, 0.2 mM ZnSO4, or 0.2 mM FeSO4. Biochemical assay and histochemical localization showed that O2(.-) accumulated in roots in response to pollutants, except that the staining of O2(.-) under NaHSO3 treatment was not detective. Cytochemical localization further indicated that the generated O2(.-) was present mainly in the root cortex, and pith cells, especially in NH4NO3- and FeSO4-treated roots. The pollutants also caused greatly accumulated H2O2 and (.)OH in aerial roots, which finally resulted in lipid peroxidation as indicated by increased malondialdehyde contents. We conclude that the F. microcarpa aerial roots are sensitive to pollutant-induced ROS and that the histochemical localization of O2(.-) via Nitrotetrazolium Blue chloride staining is not effective for detecting the effects of HSO3(-) treatment because of the treatment's bleaching effect.
Comments on Methods to Suppress Endogenous beta-Galactosidase Activity in Mouse Tissues Expressing the LacZ Reporter Gene.[Pubmed:27555495]
J Histochem Cytochem. 2016 Oct;64(10):579-86.
The Escherichia coli LacZ gene (encoding beta-galactosidase) is a widely used reporter for gene regulation analysis in transgenic mice. Determination of beta-galactosidase activity is classically performed using 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside/ferri-/ferrocyanide (X-Gal/FeCN) histochemistry. Uncertainty about the origin of the beta-galactosidase signal is encountered in tissues containing high levels of endogenous beta-galactosidase. Here, we show that reliable results can nevertheless be obtained in these tissues by performing the histochemical reaction under slightly basic pH conditions (pH 8-9). We further demonstrate that in this context, analysis of tissue sections may be advantageous over that of conventional whole-mount tissues because poor dye penetration and remaining tissue acidity are avoided in tissue sections. We also recommend that bacterial debris should always be carefully removed from the luminal surface of gastrointestinal tract specimens unless staining of resident microflora is deliberately used as an internal positive control in the assay. Finally, we show that 6-chloro-3-indolyl-beta-d-galactopyranoside with Nitrotetrazolium Blue chloride works well as an alternative chromogenic substrate for visualizing LacZ reporter gene expression in cryostat sections. Its use in high endogenous beta-galactosidase-expressing organs is superior over the use of X-Gal/FeCN at slightly basic pH conditions.


