Apigenin-7-glucuronideCAS# 29741-09-1 |
Quality Control & MSDS
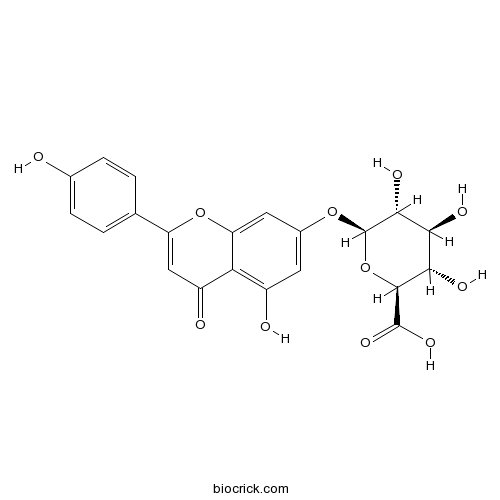
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29741-09-1 | SDF | Download SDF |
| PubChem ID | 5319484 | Appearance | Yellowish powder |
| Formula | C21H18O11 | M.Wt | 446.36 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Scutellarin A; 4',5,7-Trihydroxyflavone 7-glucuronide | ||
| Solubility | DMSO : 65 mg/mL (145.62 mM; Need ultrasonic) | ||
| Chemical Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-[5-hydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O | ||
| Standard InChIKey | JBFOLLJCGUCDQP-ZFORQUDYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Apigenin-7-glucuronide possesses multiple pharmacological activities, including anti-oxidant, anti-complement, anti-inflammatory, and aldose reductase inhibitory activities, it can inhibit Matrix Metalloproteinases (MMP) activities, with IC50s of 12.87, 22.39, 17.52, 0.27 μM for MMP-3, MMP-8, MMP-9, MMP-13, respectively. Apigenin 7-O-β-D-glucuronide protects mice from LPS-induced endotoxin shock by inhibiting proinflammatory cytokine production, it may be used as a dietary complement for health promotion. |
| Targets | NO | PGE | TNF-α | NOS | COX | AP-1 | ERK | p38MAPK | MMP-3 | MMP-8 | MMP-9 | MMP-13 |
| In vivo | Apigenin-7-O-β-D-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling pathways in RAW 264.7 macrophages and protects mice against endotoxin shock.[Pubmed: 26750400 ]Food Funct. 2016 Feb;7(2):1002-13.Apigenin-7-O-β-D-glucuronide (Apigenin-7-glucuronide,AG), an active flavonoid derivative isolated from the agricultural residue of Juglans sigillata fruit husks, possesses multiple pharmacological activities, including anti-oxidant, anti-complement, and aldose reductase inhibitory activities.
To date, no report has identified the anti-inflammatory mechanisms of AG.
|
| Structure Identification | Arch Pharm Res. 2008 Jan;31(1):28-33.Comparative antioxidant activity and HPLC profiles of some selected Korean thistles.[Pubmed: 18277604]As yet, no comparative analyses have been conducted regarding the comparative antioxidant activities and HPLC profiles of thistles distributed in Korea.
|

Apigenin-7-glucuronide Dilution Calculator

Apigenin-7-glucuronide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2403 mL | 11.2017 mL | 22.4034 mL | 44.8069 mL | 56.0086 mL |
| 5 mM | 0.4481 mL | 2.2403 mL | 4.4807 mL | 8.9614 mL | 11.2017 mL |
| 10 mM | 0.224 mL | 1.1202 mL | 2.2403 mL | 4.4807 mL | 5.6009 mL |
| 50 mM | 0.0448 mL | 0.224 mL | 0.4481 mL | 0.8961 mL | 1.1202 mL |
| 100 mM | 0.0224 mL | 0.112 mL | 0.224 mL | 0.4481 mL | 0.5601 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Teniposide
Catalog No.:BCC3864
CAS No.:29767-20-2
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Threo-methylphenidate hydrochloride
Catalog No.:BCC5818
CAS No.:298-59-9
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
- Nitrotetrazolium Blue chloride
Catalog No.:BCC6465
CAS No.:298-83-9
Comparative antioxidant activity and HPLC profiles of some selected Korean thistles.[Pubmed:18277604]
Arch Pharm Res. 2008 Jan;31(1):28-33.
As yet, no comparative analyses have been conducted regarding the comparative antioxidant activities and HPLC profiles of thistles distributed in Korea. Thus, this study was performed in order to evaluate the antioxidant potentials of seven Korean thistles: Cirsium lineare, Cirsium chanroenicum, Cirsium setidens, Cirsium japonicum var. ussuriense, Cirsium nipponicum, Cirslum pendulum and Carduus crispus, via peroxynitrite and DPPH free radical assays. Among seven Korean thistles, Carduus crispus exhibited the most significant antioxidant activity in both DPPH assay and peroxynitrite. In order to characterize the compounds contained in Korean thistles, we conducted HPLC analyses on the following ten flavonoids: luteolin-5-glucoside (1), luteolin-7-glucoside (2), apigenin-7-glucoside (3), hispidulin-7-neohesperidoside (4), Apigenin-7-glucuronide (5), cirsimarin (6), pectolinarin (7), luteolin (8), apigenin (9) and acacetin (10). The results of our HPLC analyses indicated the presence of pectolinarin in the whole plants of C. setidens, C. lineare, C. nipponicum, C. pendulum, the aerial and underground parts of C. japonicum var. ussuriense, and the aerial parts of C. chanroenicum. Moreover, we were able to identify hispidulin-7-neohesperidoside and luteolin-7-glucoside in the whole plants of Carduus crispus, acacetin in the aerial parts of C. chanroenicum, cirsimarin in C. lineare.


