TeniposideCAS# 29767-20-2 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29767-20-2 | SDF | Download SDF |
| PubChem ID | 452548 | Appearance | Powder |
| Formula | C32H32O13S | M.Wt | 656.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VM26 | ||
| Solubility | DMSO : ≥ 30 mg/mL (45.69 mM) *"≥" means soluble, but saturation unknown. | ||
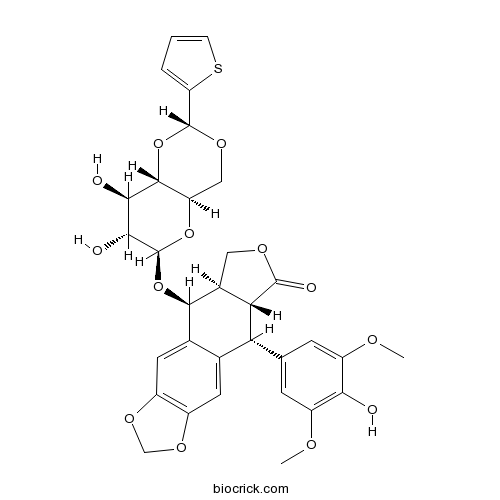
| Chemical Name | (5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-thiophen-2-yl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2C3C(COC3=O)C(C4=CC5=C(C=C24)OCO5)OC6C(C(C7C(O6)COC(O7)C8=CC=CS8)O)O | ||
| Standard InChIKey | NRUKOCRGYNPUPR-QBPJDGROSA-N | ||
| Standard InChI | InChI=1S/C32H32O13S/c1-37-19-6-13(7-20(38-2)25(19)33)23-14-8-17-18(42-12-41-17)9-15(14)28(16-10-39-30(36)24(16)23)44-32-27(35)26(34)29-21(43-32)11-40-31(45-29)22-4-3-5-46-22/h3-9,16,21,23-24,26-29,31-35H,10-12H2,1-2H3/t16-,21+,23+,24-,26+,27+,28+,29+,31+,32-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Teniposide is a podophyllotoxin derivative, acts as a topoisomerase II inhibitor, and used as a chemotherapeutic agent.In Vitro:Teniposide is a topoisomerase II inhibitor. Teniposide (VM-26, 0.15-45 mg/L) inhibits the proliferation of Tca8113 cells in a dose-dependent manner, with an IC50 of 0.35 mg/L. Teniposide (5 mg/L) induces apoptosis of Tca8113 cells. Teniposide (5.0 mg/L) causes cell arrested at G2/M phase in Tca8113 cells[2]. Teniposide is active on primary cultured glioma cells from patients, when the level of miR-181b is high in the cells, with an IC50 of 1.3 ± 0.34 μg/mL. Cells treated with teniposide with low MDM2 have decreased viability compared with control cells, and the IC50 decreases from 5.86 ± 0.36 μg/mL to 2.90 ± 0.35 μg/mL upon MDM2 suppression. Teniposide also inhibits the viability of glioma cell with high level of miR-181b, through mediation of MDM2[3].In Vivo:Teniposide (0.5 mg/kg, i.p.) significantly increases micronucleated polychromatic erythrocyte (MNPCE) frequencies, which is directly related to bone marrow toxicity as significant suppression of bone marrow is noted. Teniposide (24 mg/kg, i.p.) markedly decreases the frequencies of BrdU-labelled sperm. Teniposide (12, 24 mg/kg, i.p.) also dramatically induces disomic sperm in the germ cell of male mice[1]. References: | |||||

Teniposide Dilution Calculator

Teniposide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5228 mL | 7.6138 mL | 15.2277 mL | 30.4553 mL | 38.0691 mL |
| 5 mM | 0.3046 mL | 1.5228 mL | 3.0455 mL | 6.0911 mL | 7.6138 mL |
| 10 mM | 0.1523 mL | 0.7614 mL | 1.5228 mL | 3.0455 mL | 3.8069 mL |
| 50 mM | 0.0305 mL | 0.1523 mL | 0.3046 mL | 0.6091 mL | 0.7614 mL |
| 100 mM | 0.0152 mL | 0.0761 mL | 0.1523 mL | 0.3046 mL | 0.3807 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Teniposide is a glycoside-derivative of podophyllotoxin. Has cytostatic effect. Inhibits topoisomerase II while induces apoptosis.
- Altenuene
Catalog No.:BCN7392
CAS No.:29752-43-0
- Altretamine hydrochloride
Catalog No.:BCC4114
CAS No.:2975-00-0
- Loganetin
Catalog No.:BCN5202
CAS No.:29748-10-5
- Luteolin-7-O-glucuronide
Catalog No.:BCN5338
CAS No.:29741-10-4
- Apigenin-7-glucuronide
Catalog No.:BCN5326
CAS No.:29741-09-1
- Dihydro-β-erythroidine hydrobromide
Catalog No.:BCC7341
CAS No.:29734-68-7
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- Nudifloside B
Catalog No.:BCN7474
CAS No.:297740-98-8
- Nudifloside C
Catalog No.:BCN7491
CAS No.:297740-99-9
- Silydianin
Catalog No.:BCN2388
CAS No.:29782-68-1
- Carbamazepine
Catalog No.:BCC4378
CAS No.:298-46-4
- Threo-methylphenidate hydrochloride
Catalog No.:BCC5818
CAS No.:298-59-9
- Xanthotoxin
Catalog No.:BCN5205
CAS No.:298-81-7
- Nitrotetrazolium Blue chloride
Catalog No.:BCC6465
CAS No.:298-83-9
- MTT
Catalog No.:BCC8031
CAS No.:298-93-1
- Desmethylbellidifolin
Catalog No.:BCN3868
CAS No.:2980-32-7
- H-Pro-NMe2
Catalog No.:BCC3019
CAS No.:29802-22-0
- Fatostatin A
Catalog No.:BCC6184
CAS No.:298197-04-3
- Shanzhiside
Catalog No.:BCN5203
CAS No.:29836-27-9
A Highly Efficient Approach To Construct (epi)-Podophyllotoxin-4-O-glycosidic Linkages as well as Its Application in Concise Syntheses of Etoposide and Teniposide.[Pubmed:26916150]
Org Lett. 2016 Mar 18;18(6):1294-7.
By taking full advantage of the mild promotion conditions of an ortho-alkynylbenzoate glycosylation protocol, a highly efficient approach to construct the challenging (epi)-podophyllotoxin 4-O-glycosidic linkages was devised under the activation of a catalytic amount of a Au(I) complex. The novel method enjoys a quite broad substrate scope in terms of both glycosyl donors and podophyllotoxin derivative acceptors, providing the desired glycosides in excellent yields. Based on the new approach, concise syntheses of clinically used anticancer reagents etoposide and Teniposide were accomplished, and the overall yields counting from easily available starting materials could reach as high as 18% and 9%, respectively.
Cremophor-free intravenous self-microemulsions for teniposide: Safety, antitumor activity in vitro and in vivo.[Pubmed:26253377]
Int J Pharm. 2015 Nov 10;495(1):144-153.
The study was designed to identify the safety and antitumor activity of Teniposide self-microemulsified drug delivery system (TEN-SMEDDS) previously developed, and to provide evidence for the feasibility and effectiveness of TEN-SMEDDS for application in clinic. The TEN-SMEDDS could form fine emulsion with mean diameter of 279 +/- 19 nm, Zeta potential of -6.9 +/- 1.4 mV, drug loading of 0.04 +/- 0.001% and entrapment efficiency of 98.7 +/- 1.6% after dilution with 5% glucose, respectively. The safety, including hemolysis, hypersensitivity, vein irritation and toxicity in vivo, and antitumor activity were assessed, VUNON as a reference. Sulforhodamine B assays demonstrated that the IC50 of TEN-SMEDDS against C6 and U87MG cells were higher than that of VUMON. But the effect of TEN-SMEDDS on the cell cycle distribution and cell apoptotic rate was similar to that of VUMON as observed by flow cytometry. Likewise, the antitumor activity of TEN-SMEDDS was considerable to that of VUMON. Finally, the TEN-SMEDDS exhibited less body weight loss, lower hemolysis and lower myelosuppression as compared with VUMON. In conclusion, promising TEN-SMEDDS retained the antitumor activity of Teniposide and was less likely to cause some side effects compared to VUMON. It may be favorable for the application in clinic.
Preparation and evaluation of teniposide-loaded polymeric micelles for breast cancer therapy.[Pubmed:27596115]
Int J Pharm. 2016 Nov 20;513(1-2):118-129.
Self-assembled polymeric micelles have been widely applied in anticancer drug delivery systems. Teniposide is a broad spectrum and effective anticancer drug, but its poor water-solubility and adverse effects of commercial formulation (VM-26) restrict its clinical application. In this work, Teniposide-loaded polymeric micelles were prepared based on monomethoxy-poly(ethylene glycol)-poly(epsilon-caprolactone-co-d,l- lactide) (MPEG-PCLA) copolymers through a thin-film hydration method to improve the hydrophilic and reduce the systemic toxicity. The prepared Teniposide micelles were without any surfactants or additives and monodisperse with a mean particle size of 29.6+/-0.3nm. The drug loading and encapsulation efficiency were 18.53+/-0.41% and 92.63+/-2.05%, respectively. The encapsulation of Teniposide in MPEG-PCLA micelles showed a slow and sustained release behavior of Teniposide in vitro and improved the terminal half-life (t1/2), the area under the plasma concentration-time curve (AUC) and retention time of Teniposide in vivo compared with VM-26. In addition, Teniposide micelles also enhanced the cellular uptake by MCF-7 breast cancer cells in vitro and increased the distribution in tumors in vivo. Teniposide micelles showed an excellent safety with a maximum tolerated dose (MTD) of approximately 50mg Teniposide/kg body weight, which was 2.5-fold higher than that of VM-26 (about 20mg Teniposide/kg body weight). Furthermore, the intravenous application of Teniposide micelles effectively suppressed the growth of subcutaneous MCF-7 tumor in vivo and exhibited a stronger anticancer effect than that of VM-26. These results suggested that we have successfully prepared Teniposide-loaded MPEG-PCLA micelles with improved safety, hydrophilic and therapeutic efficiency, which are efficient for Teniposide delivery. The prepared Teniposide micelles may be promising in breast cancer therapy.
Patients treatment with neuroglioma by teniposide and semustine and its influence on Twist and E-cadherin expression.[Pubmed:27275118]
Saudi Pharm J. 2016 May;24(3):299-304.
UNLABELLED: This study focuses on curative effects of Teniposide combining with semustine on patients with neuroglioma and the influences on the expression of Twist and E-cadherin in tissue. Sixty-eight patients with neuroglioma taking operation in our hospital were divided into two groups randomly. Single radiotherapy was given to 34 patients in group A, and Teniposide (VM-26) and semustine (Me-CCUN) were added to radiotherapy for 34 patients in group B. Then, curative effects, survival rate, living quality and adverse reaction rate after operation were compared between two groups. Moreover, the difference in positive expression rate of Twist and E-cadherin before and after treatment between two groups was analyzed by immunohistochemistry. RESULTS: In group B, the effective rate of treatment was 88.2%, and the disease control rate was 70.6%, higher than 52.9% and 32.4% in group A with statistical significance (P < 0.05). Moreover, the survival rate in three years of group B was 44.1%, and the score of living quality was 67.11 +/- 4.32, and also higher than 23.5% and 63.79 +/- 4.53 in group A with statistical significance (P < 0.05). However, the difference between two groups in adverse reaction rate has no statistical significance (P > 0.05). In addition, the difference in positive expression rate of Twist and E-cadherin between group A and group B has no statistical significance before treatment (P > 0.05). After treatment, however, the positive rate of Twist in group B is lower than that in group A, while the positive rate of E-cadherin is higher. Both differences have statistical significance (P < 0.05). Chemotherapy of VM-26 combining with Me-CCNU can inhibit Twist expression and improve the expression rate of E-cadherin to help improving the curative effects and living quality and increasing survival rate.


